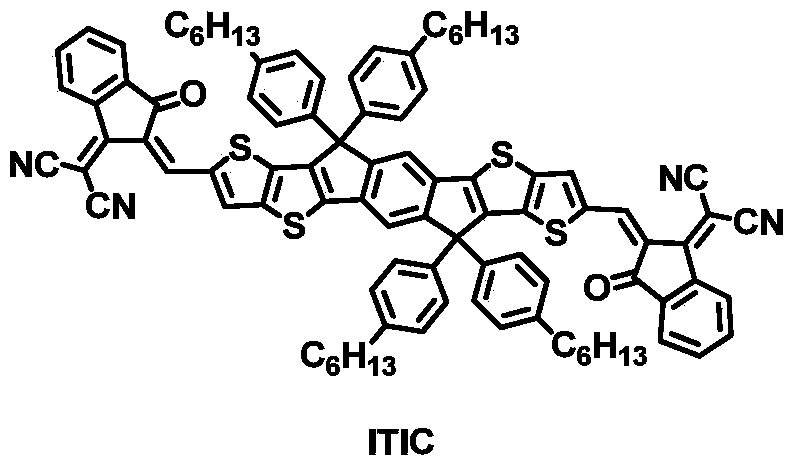
A silane-substituted two-dimensional conjugated polymer and its preparation method and application in photovoltaic devices
A two-dimensional conjugated, polymer technology, used in photovoltaic power generation, electric solid-state devices, semiconductor devices, etc., can solve the problems of poor morphology stability, low efficiency, weak absorption in the visible light region, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0084] Example 1, 2,6-bis(trimethyltin group) 4,8-bis((3-n-propyl)silthienyl)benzo[1,2-b:4,5-b']di Synthesis of Thiophene (M1) Compound
[0085]
[0086] According to the reaction equation shown above, under the protection of argon, slowly add n-butyllithium (40 ml, 2.5M / L, Stored in n-hexane), the mixture was kept at -78° for 1 hour and slowly warmed to room temperature. Then, tripropylsilyl chloride (19.2 g, 100 mmol) was added, and the mixture was stirred overnight. The mixture was extracted twice with ether, washed with distilled water and brine. Further purification by silica gel column chromatography using hexane as eluent gave 2-(3-propylsilyl)thiophene (A01) as a colorless liquid.
[0087] The structural confirmation data are as follows: GC-MS: m / z=240, 1 HNMR (400MHz, CDCl 3 ),δ(ppm):7.57-7.56(d,1H),7.22(d,1H),7.16-7.15(t,1H),1.40-1.32(m,6H),0.96-0.92(m,9H), 0.80-0.75(m,6H).
[0088] Add 7.20 g (30.0 mmol) of 2-(3-propylsilyl)thiophene (A01) into a 250 mL tw...
Embodiment 2
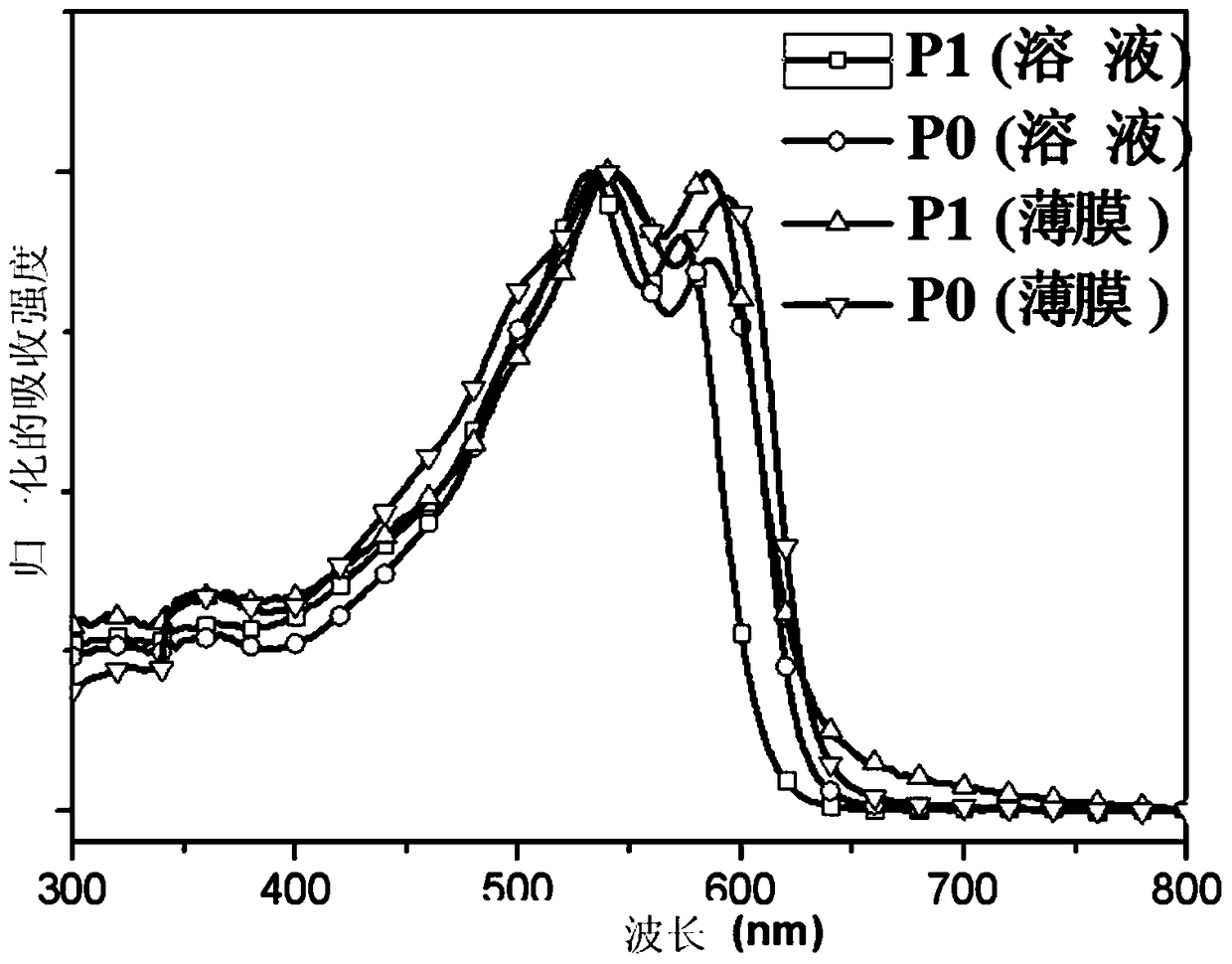
[0091] Embodiment 2, formula P 1 Synthesis of the indicated polymers
[0092]
[0093] Carry out according to the above reaction equation, take 0.3mmol each of monomer M1 and M2, dissolve it in a mixed solvent of toluene (8mL) and DMF (2mL), exhaust the air with argon for 5 minutes, and then add catalyst tetrakis (triphenyl Phosphine)palladium(0) (20 mg) was followed by further evacuation of air for 25 minutes, and then the polymerization was stopped after 12 hours at the reflux temperature of toluene. The polymer solution was cooled to room temperature, and slowly precipitated into methanol (50 mL), and the precipitated solid polymer was sequentially eluted with methanol and n-hexane in a Soxhlet extractor. Finally dissolve with chloroform and precipitate into methanol, filter, and vacuum dry for one day to obtain the formula P of black solid powder 1 The polymer shown, the productive rate is 81%, GPC: Mn=23.5K; Mw / Mn=2.0.Anal.Calcd for C 66 h 85 f 2 N 3 S 6 Si 2 (...
Embodiment 3
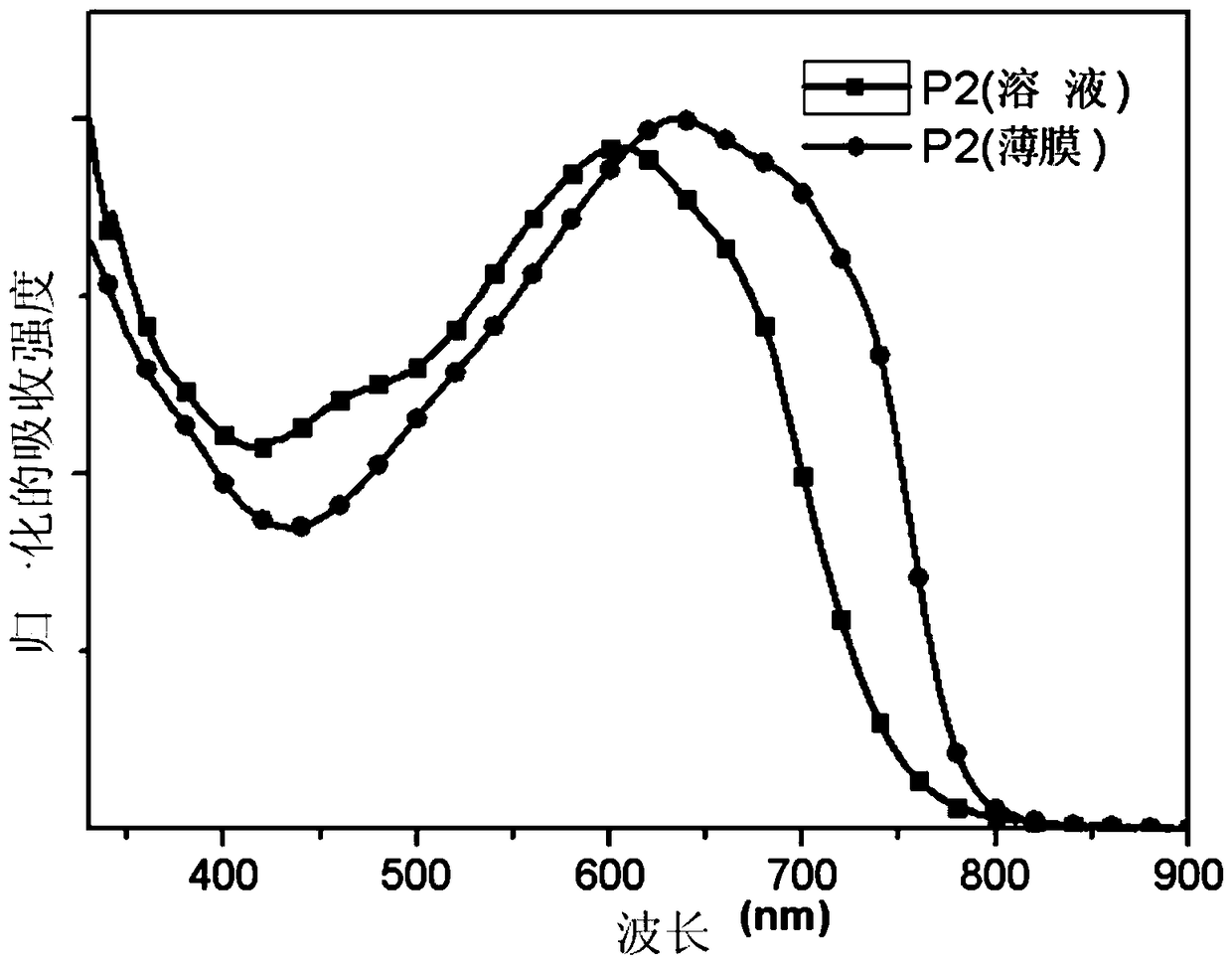
[0094] Embodiment 3, formula P 2 Synthesis of the indicated polymers
[0095]
[0096] According to the above reaction equation, take 0.3mmol of each monomer M1 and M3, dissolve it in a mixed solvent of toluene (8mL) and DMF (2mL), exhaust the air with argon for 5 minutes, and then add the catalyst tetrakis (triphenyl Phosphine)palladium(0) (20 mg) was followed by further evacuation of air for 25 minutes, and then the polymerization was stopped after 12 hours at the reflux temperature of toluene. The polymer solution was cooled to room temperature, and slowly precipitated into methanol (50 mL), and the precipitated solid polymer was sequentially eluted with methanol and n-hexane in a Soxhlet extractor. Finally dissolve with chloroform and precipitate into methanol, filter, and vacuum dry for one day to obtain the formula P of black solid powder 1 The polymer shown, the productive rate is 65%, GPC: Mn=18.2K; Mw / Mn=1.9.Anal.Calcd for C 51 h 65 FOS 6 Si 2 (%): C, 63.70; ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com