Cardanol-based antioxidant as well as preparation method and application thereof
A cardanol-based, antioxidant technology is applied in the preparation of amino hydroxy compounds, chemical instruments and methods, preparation of organic compounds, etc. The effect of easy handling, reduced pollution, and simple and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0048] Another aspect of the present invention provides a method for preparing the cardanol-based antioxidant, comprising: taking cardanol and / or cardanol derivatives, aromatic amines and / or aromatic amine derivatives in a liquid phase system with The coupling reagent reacts to obtain the cardanol-based antioxidant. In some embodiments, the preparation method of the cardanol-based antioxidant includes: taking cardanol and / or cardanol derivatives, aromatic amines and / or coupling reagents in the liquid phase reaction system, The reaction is carried out under the condition of a catalyst to obtain the cardanol-based antioxidant.
[0049] In some embodiments, the preparation method specifically includes: taking cardanol and / or cardanol derivatives, aldehydes, arylamines and / or arylamine derivatives and / or amino aromatic heterocycles and / or amino aromatic heterocycles The ring derivatives, coupling reagents and catalysts are mixed and reacted in a liquid phase reaction medium to ob...
Embodiment 1
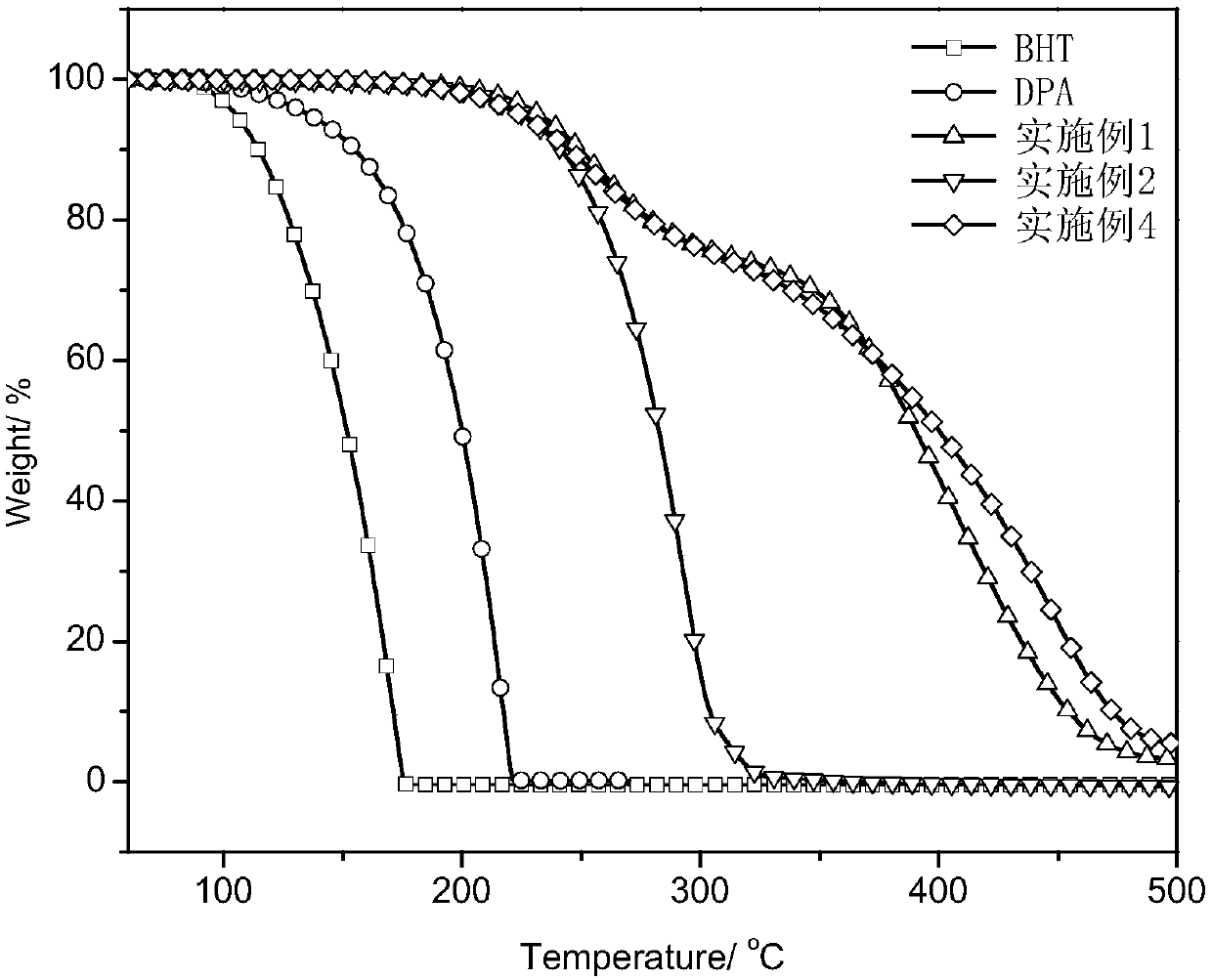
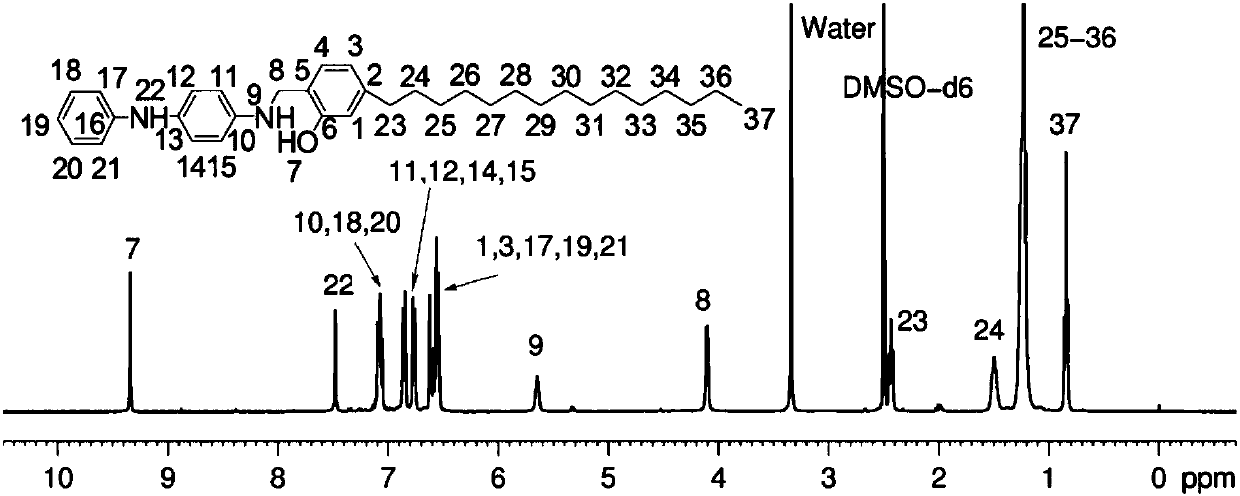
[0071] Example 1: After fully drying the 250ml three-necked reaction flask, the reactant hydrogenated cardanol (3.04g), paraformaldehyde (0.45g), p-aminodiphenylamine (1.84g), and reaction solvent methanol (50ml) were added successively , the temperature was raised to 85° C., and the reaction was stirred for 5 hours, and water was removed during the reaction. Then, it was washed and filtered with methanol, and the filter cake was recrystallized three times with ethyl acetate, and after drying, 4.5 g of a light yellow solid product was obtained. of the product 1 H NMR spectrum (DMSO, 400MHz) and 13 C NMR spectrum (DMSO, 100MHz) can be found in figure 2 with image 3 .
Embodiment 2
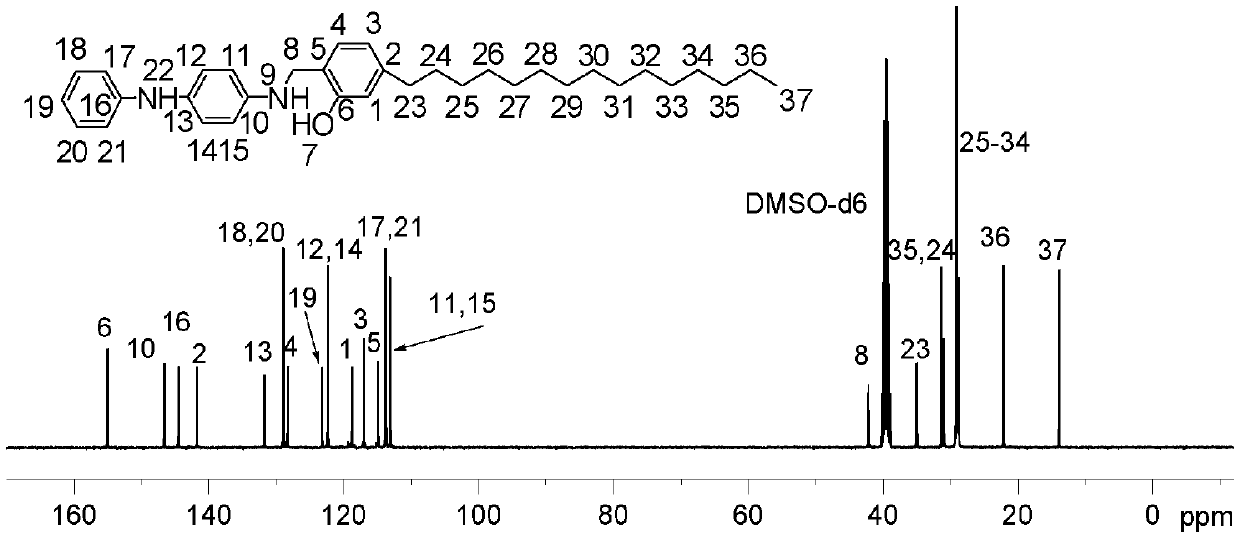
[0072] Embodiment 2: After fully drying the three-necked reaction flask of 250ml, add reactant hydrogenated cardanol (6.08g), paraformaldehyde (0.75g), p-phenylenediamine (1.1g), reaction solvent methanol (70ml) successively, The temperature was raised to 85° C., the reaction was stirred for 5 hours, and water was removed during the reaction. Then, it was washed and filtered with methanol, and the filter cake was recrystallized three times with ethyl acetate, and after drying, 6.8 g of a light-colored solid product was obtained. of the product 1 H NMR spectrum (DMSO, 400MHz) and 13 C NMR spectrum (DMSO, 100MHz) can be found in Figure 4 with Figure 5 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com