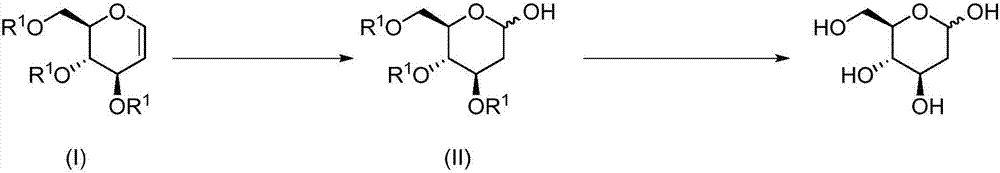
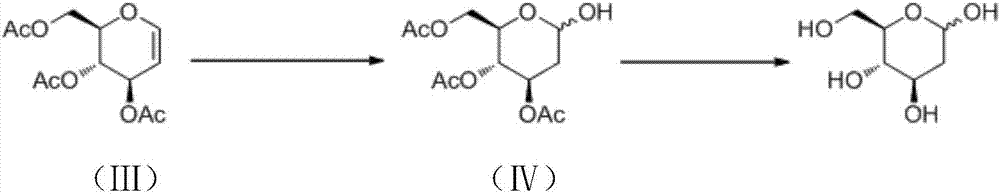
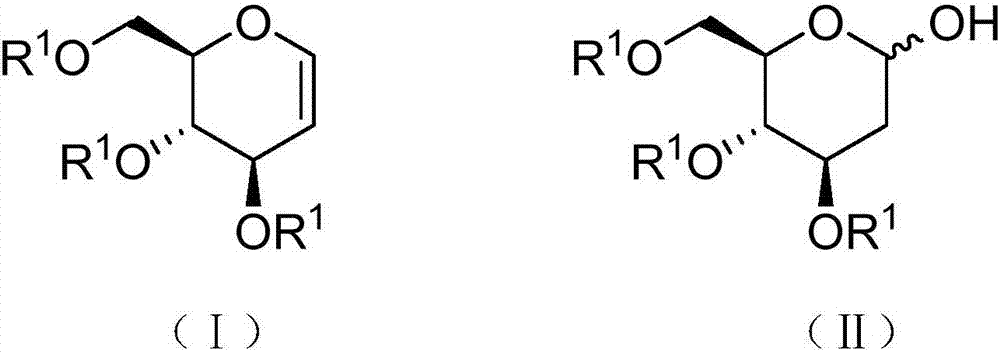Preparation method of 2-deoxy-D-glucose
A glucose and compound technology, applied in the field of preparation of 2-deoxy-D-glucose, can solve the problems of a large amount of strong acid wastewater, inconvenient use, difficult commercialization, etc., and achieve broad commercial application prospects. rate-enhancing effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] A preparation method of 2-deoxy-D-glucose, comprising the following steps:
[0029] (1) To a solution of compound (III) (27.2 g, 0.1 mol) in acetonitrile (500 mL) was added CH at room temperature 3 SCH 2 Cl (3.7 g, 0.02 mol), KI (3.3 g, 0.02 mol) and water (5.4 g, 0.3 mol). After stirring at room temperature for 10 min, ethyl acetate (250 mL) and saturated sodium thiosulfate solution (150 mL) were added to the reaction solution, and the organic phase was washed with water, saturated brine, dried and concentrated under reduced pressure. The crude product was recrystallized from diethyl ether (150 mL) to obtain compound (IV) (29.2 g, white solid) with a yield of 99%.
[0030] Hydrogen NMR spectrum: (600MHz, CDCl 3 )δ5.54-5.35(m, 8H), 5.08-4.95(m, 6H), 4.37-4.19(m, 10H), 4.13(t, J=13.3Hz, 5H), 3.69(d, J=6.9Hz ,1H),3.49(s,1H),3.04(s,4H),2.42(dd,J=11.9,4.1Hz,1H),2.32-2.27(m,4H),2.14-2.00(m,45H), 1.88-1.73 (m, 5H).
[0031] (2) Dissolve compound (IV) (20 g, 0.069 mol) i...
Embodiment 2
[0036] A preparation method of 2-deoxy-D-glucose, comprising the following steps:
[0037] (1) PhCH was sequentially added to a solution of compound (V) (41.6 g, 0.1 mol) in acetonitrile (500 mL) at room temperature 2 SCH 2 Cl (1.22 g, 0.01 mol), potassium iodide (1.65 g, 0.01 mol) and water (5.4 g, 0.3 mol). After stirring at room temperature for 1 hour, ethyl acetate (250 mL) and saturated sodium thiosulfate solution (150 mL) were added to the reaction solution, and the organic phase was washed with water, saturated brine, dried and concentrated under reduced pressure. The crude product was recrystallized from diethyl ether (150 mL) to obtain compound (VI) (38.7 g, white solid) in a yield of 89%.
[0038] Hydrogen NMR spectrum: 600MHz, CDCl 3 )δ7.36-7.24(m,65H),7.17(d,J=6.0Hz,10H),5.38(s,4H),4.88(t,J=11.0Hz,5H),4.70-4.60(m,10H) ),4.57(dd,J=12.1,6.5Hz,6H),4.51(dd,J=12.1,4.6Hz,10H),4.04(dt,J=7.7,7.0Hz,9H),3.76-3.61(m, 10H), 3.61-3.54(m, 1H), 3.46-3.41(m, 4H), 3.43(dd, J=1...
Embodiment 3
[0044] A preparation method of 2-deoxy-D-glucose, comprising the following steps:
[0045] (1) To a solution of compound (III) (27.2 g, 0.1 mol) in acetonitrile (500 mL) was added n-C at room temperature 6 H 13 SCH 2 Cl (1.65 g, 0.01 mol), KI (1.65 g, 0.01 mol) and water (5.4 g, 0.3 mol). After stirring at room temperature for 1 hour, ethyl acetate (250 mL) and saturated sodium thiosulfate solution (150 mL) were added to the reaction solution, and the organic phase was washed with water, saturated brine, dried and concentrated under reduced pressure. The crude product was recrystallized from diethyl ether (150 mL) to obtain compound (IV) (25.1 g, white solid) with a yield of 85%.
[0046] Hydrogen NMR spectrum: (600MHz, CDCl 3 )δ5.54-5.35(m, 8H), 5.08-4.95(m, 6H), 4.37-4.19(m, 10H), 4.13(t, J=13.3Hz, 5H), 3.69(d, J=6.9Hz ,1H),3.49(s,1H),3.04(s,4H),2.42(dd,J=11.9,4.1Hz,1H),2.32-2.27(m,4H),2.14-2.00(m,45H), 1.88-1.73 (m, 5H).
[0047] (2) This step is the same as step (2)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com