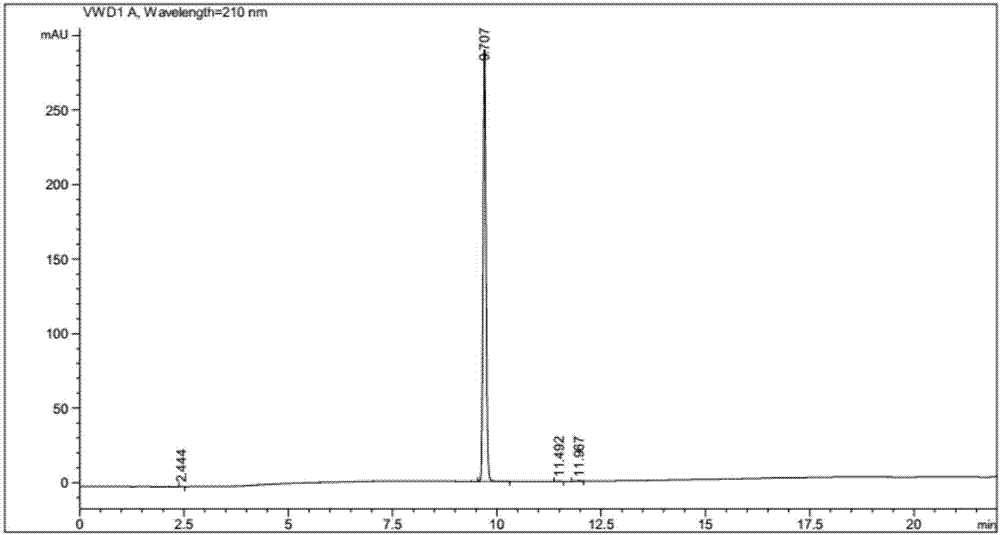
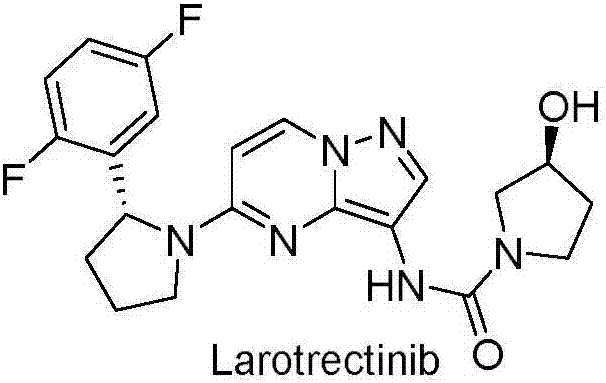
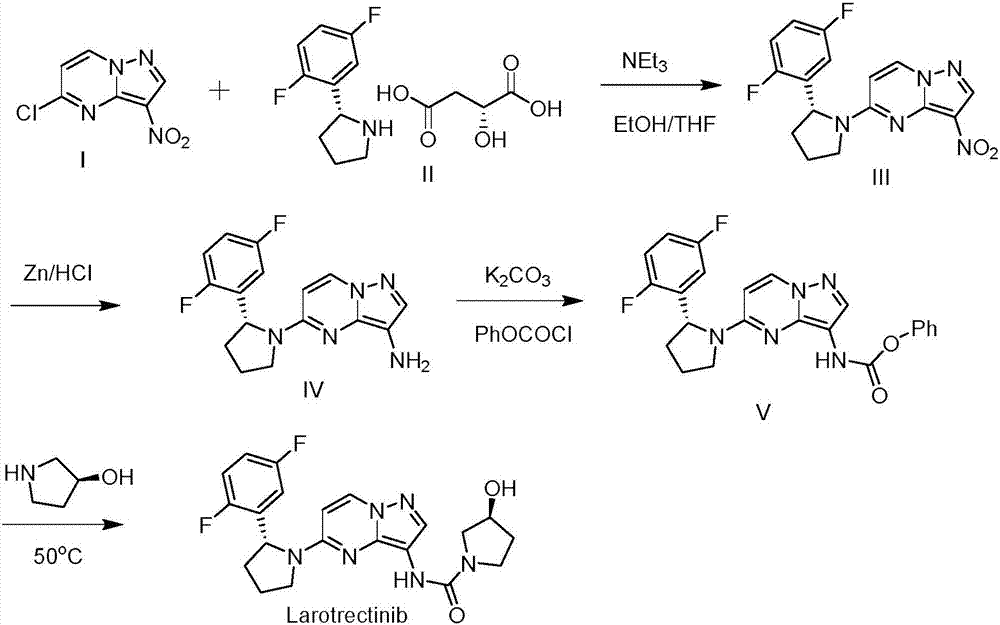
Preparation method of Larotrectinib and intermediate of Larotrectinib
A system and compound technology, applied in the field of medicine and chemical industry, can solve the problems of low utilization of formula II compound, high industrialization cost, complicated purification operation, etc., and achieve the effects of shortening reaction time, reducing types and contents, and reducing reaction temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Embodiment 1: the synthesis of formula 2 compound
[0036]
[0037] Add the compound of formula 1 (50g, 250mmol), ethanol (500mL), ammonium chloride (133g, 2.5mol) aqueous solution (500mL) and iron powder (140g, 2.5mol) into the reaction flask, heat, and reflux under nitrogen protection Under reaction 4-8 hours. After the reaction was completed, it was concentrated under reduced pressure, water and dichloromethane were added, and the layers were separated. The organic phase was washed with saturated brine, dried over anhydrous sodium sulfate, and concentrated under reduced pressure to obtain 40 g of the compound of formula 2 with a yield of 95.2%. δ=9.29(d,J=7.2Hz,1H),8.71(s,1H),8.16(d,J=7.2Hz,1H),5.92(s,2H); MS(m / z)[M+H ]+calcd for C 6 h 6 ClN 4 169.0, found 169.8.
Embodiment 2
[0038] Embodiment 2: the synthesis of formula 3 compound
[0039]
[0040] Add the compound of formula 2 (40g, 237mmol), N,N-diisopropylethylamine (36.8g, 284mmol) and methylene chloride (400mL) into the reaction flask at room temperature, drop to 0-10°C, slowly Add a dichloromethane solution of p-nitrophenyl chloroformate (50.2g, 249mmol) dropwise, keep the temperature not exceeding 10°C, and control in TLC. After the reaction is completed, wash with saturated brine, dry with anhydrous sodium sulfate, and Concentration under reduced pressure gave 74 g of the compound of formula 3 with a yield of 93.5%. 1H NMR (300MHz, d 6 DMSO)δ=10.2(s,1H),9.32(d,J=7.2Hz 1H),8.81(s,1H),7.4-8.6(m,5H); MS(m / z)[M+H]+ calcd for C 13 h 9 ClN 5 o 4 334.0, found 333.8.
Embodiment 3
[0041] Embodiment 3: the synthesis of formula 5 compounds
[0042]
[0043] Add the compound of formula 3 (70g, 210mmol), the compound of formula 4 (18g, 207mmol) and ethanol (300mL) into the reaction flask at room temperature, react at 25°C for 2-8 hours, control in TLC, after the reaction is completed, stir Methyl tert-butyl ether (800mL) was added under low temperature, and solids were precipitated. Continue stirring at this temperature for 30 minutes, filter, and drain to obtain 36g of the compound of formula 5. The mother liquor was recovered and purified by column chromatography to obtain 18g of the compound of formula 5. A total of 54g, the yield is 91%. 1H NMR (300MHz, d 6DMSO) δ=9.26(d,J=7.2Hz 1H),8.73(s,1H),8.78(s,1H),8.07(d,J=7.2Hz,1H),4.02(m,1H),3.62( m,2H),3.48(m,2H),2.36(m,2H); MS(m / z)[M+H]+calcd for C 11 h 13 ClN 5 o 2 282.1, found 281.9.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com