Preparation method of tiglic aldehyde derivative
A derivative, tiglic aldehyde technology, applied in the field of preparation of tiglic aldehyde derivatives, can solve the problems of poor product yield, difficult post-processing, high reaction temperature, etc., so as to save equipment cost and production cost, and reduce equipment cost. Equipment and operators, the effect of simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039]Synthesis of chlorotiglic aldehyde:
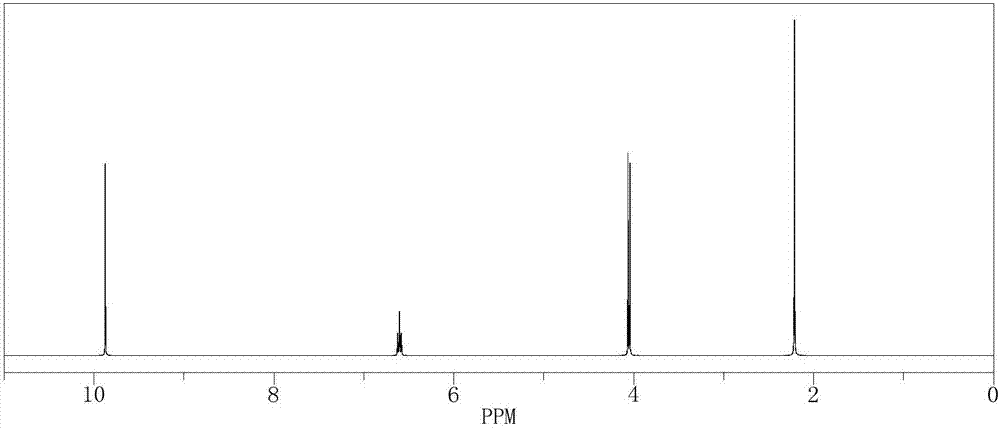
[0040] In a 500mL reactor, put 10.66g (0.1mol) of 2-methyl-4-chloro-2-butene, 210g of isopropanol aqueous solution, 0.11g of formaldehyde and SeO 2 2.1g, stir evenly, then heat up to 50°C, keep stirring under normal pressure for 5 hours, after the reaction is complete, the reaction solution is cooled to room temperature, and after standing to separate layers, the oil phase is at a reflux ratio of 2:1, and the top pressure is Absolute pressure 100Pa, the rectifying column that total number of plates is 80 separates and obtains chloro tiglic aldehyde 86.6g (GC analysis, purity is 98%), and reaction yield is 71.5%.
[0041] The 1H-NMR spectrogram of gained product chloro tiglic aldehyde is as follows figure 1 shown.
Embodiment 2
[0043] Synthesis of bromotiglic aldehyde:
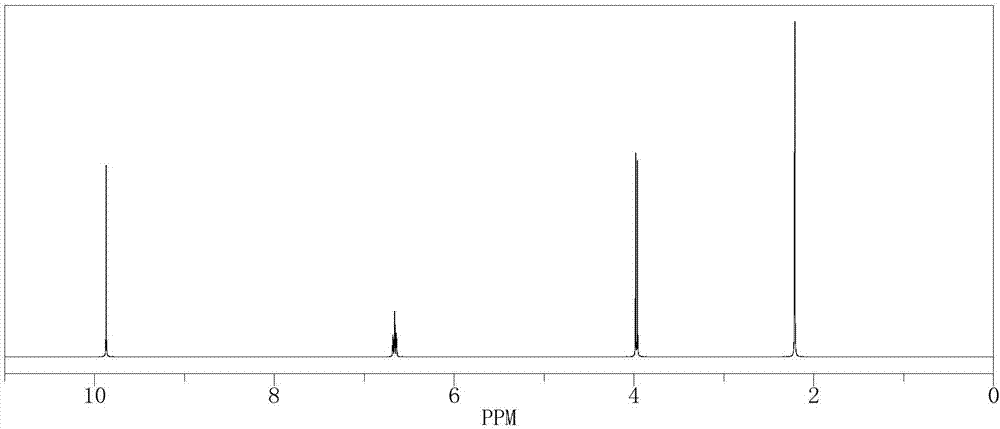
[0044] In a 500mL reactor, put 15.11g (0.1mol) of 2-methyl-4-bromo-2-butene, 210g of isopropanol aqueous solution, 0.11g of formaldehyde and SeO 2 2.1g, stir evenly, then heat up to 55°C, keep stirring under normal pressure for 4 hours, after the reaction is complete, the reaction solution is cooled to room temperature, and after standing to separate layers, the oil phase is at a reflux ratio of 2:1, and the top pressure is Absolute pressure 100Pa, the rectifying column that total number of plates is 80 separates and obtains bromo tiglic aldehyde 130.3g (GC analysis, purity is 98%), and the reaction yield is 78.3%.
[0045] The 1H-NMR spectrogram of the resulting product bromotiglic aldehyde is shown in figure 2 shown. :
Embodiment 3
[0047] Synthesis of γ-phenyl tiglic aldehyde:
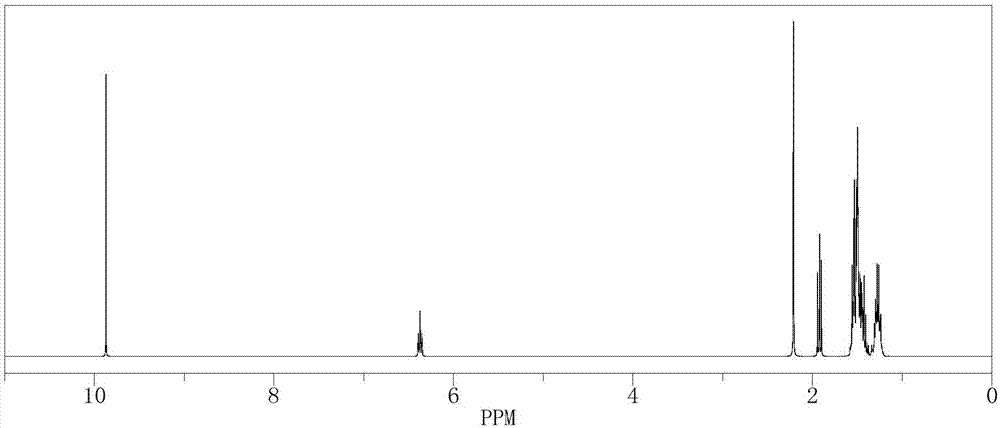
[0048] In a 500mL reactor, put 14.82g (0.1mol) of 2-methyl-4-phenyl-2-butene, 210g of isopropanol aqueous solution, 0.11g of formaldehyde and SeO 2 2.1g, stir evenly, then heat up to 60°C, keep stirring under normal pressure for 4 hours, after the reaction is complete, the reaction solution is cooled to room temperature, and after standing to separate layers, the oil phase is at a reflux ratio of 2:1, and the top pressure is The absolute pressure was 100 Pa, and the rectification tower with a total plate number of 80 was separated to obtain 115.6 g of γ-phenyl tiglic aldehyde (GC analysis, purity 98.8%), and the reaction yield was 71.3%.
[0049] The 1H-NMR spectrum of the resulting product γ-phenyl tiglic aldehyde is shown in image 3 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com