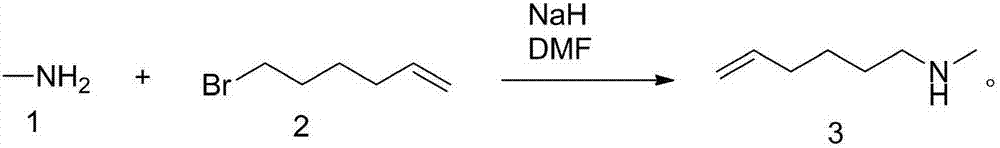
Synthesizing process of N-methyl-5-hexene-1-amine
A synthesis process, a technology of hexene, applied in the preparation of amino-substituted functional groups, organic chemistry, etc., can solve the problems of potential safety hazards and low hydrogen explosion limit, and achieve the effects of eliminating potential safety hazards, simple post-processing and high product purity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Embodiment 1: a kind of synthetic technique of N-methyl-5-hexen-1-amine, comprises the following steps: adding mass fraction in reaction bottle is the methylamine methanol solution of 25%, the content of methylamine is 2 equivalents , then add 1 equivalent of 6-bromo-1-hexene to the reaction flask, stir, heat the oil bath to 40°C, react for 3 hours, cool down to 20°C, add 0.1 equivalent of stabilizer, the stabilizer is p-formaldehyde Oxyphenol, add 1 equivalent of sodium hydroxide, recover excess methylamine methanol solution under negative pressure, heat to 50°C, and distill under reduced pressure to obtain a colorless liquid, which is N-methyl-5-hexene- 1-amine, the yield is 63%, and the purity is 95%; the synthesis process is carried out according to the following reaction formula:
[0027]
Embodiment 2
[0028] Embodiment 2: a kind of synthetic technique of N-methyl-5-hexene-1-amine, differs from embodiment 1, comprises the following steps: adding mass fraction in reaction bottle is the methylamine of 25% Methanol solution, the content of methylamine is 2 equivalents, then add 1 equivalent of 6-bromo-1-hexene to the reaction flask, stir, heat the oil bath to 45 °C, react for 2.5 hours, cool to 20 °C, add 0.1 Equivalent stabilizer, the stabilizer is p-methoxyphenol, add 1 equivalent of sodium hydroxide, recover excess methylamine methanol solution under negative pressure, heat to 55 ° C, and distill under reduced pressure to obtain a colorless liquid, which is N-methyl-5-hexen-1-amine, yield 64%, purity 95.2%.
Embodiment 3
[0029] Embodiment 3: a kind of synthetic technique of N-methyl-5-hexene-1-amine, differs from embodiment 1 in that, comprises the following steps: adding mass fraction in reaction bottle is the methylamine of 25% Methanol solution, the content of methylamine is 2 equivalents, then add 1 equivalent of 6-bromo-1-hexene to the reaction flask, stir, heat the oil bath to 50 ° C, react for 2 hours, cool down to 20 ° C, add 0.1 Equivalent stabilizer, the stabilizer is p-methoxyphenol, add 1 equivalent of sodium hydroxide, recover excess methylamine methanol solution under negative pressure, heat to 60°C, and distill under reduced pressure to obtain a colorless liquid, which is N-methyl-5-hexen-1-amine, yield 62%, purity 95.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com