Method for removing acetic acid, production method for cyclohexyl acetate, and production method for cyclohexanol
A technology of cyclohexyl acetate and cyclohexanol, which is applied in the preparation of carboxylic acid esters, chemical instruments and methods, purification/separation of hydrocarbons, etc., can solve problems such as adverse effects of reactions and equipment corrosion, and achieve good ability to capture acetic acid , to avoid corrosion, the effect of a wide range of raw material sources
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
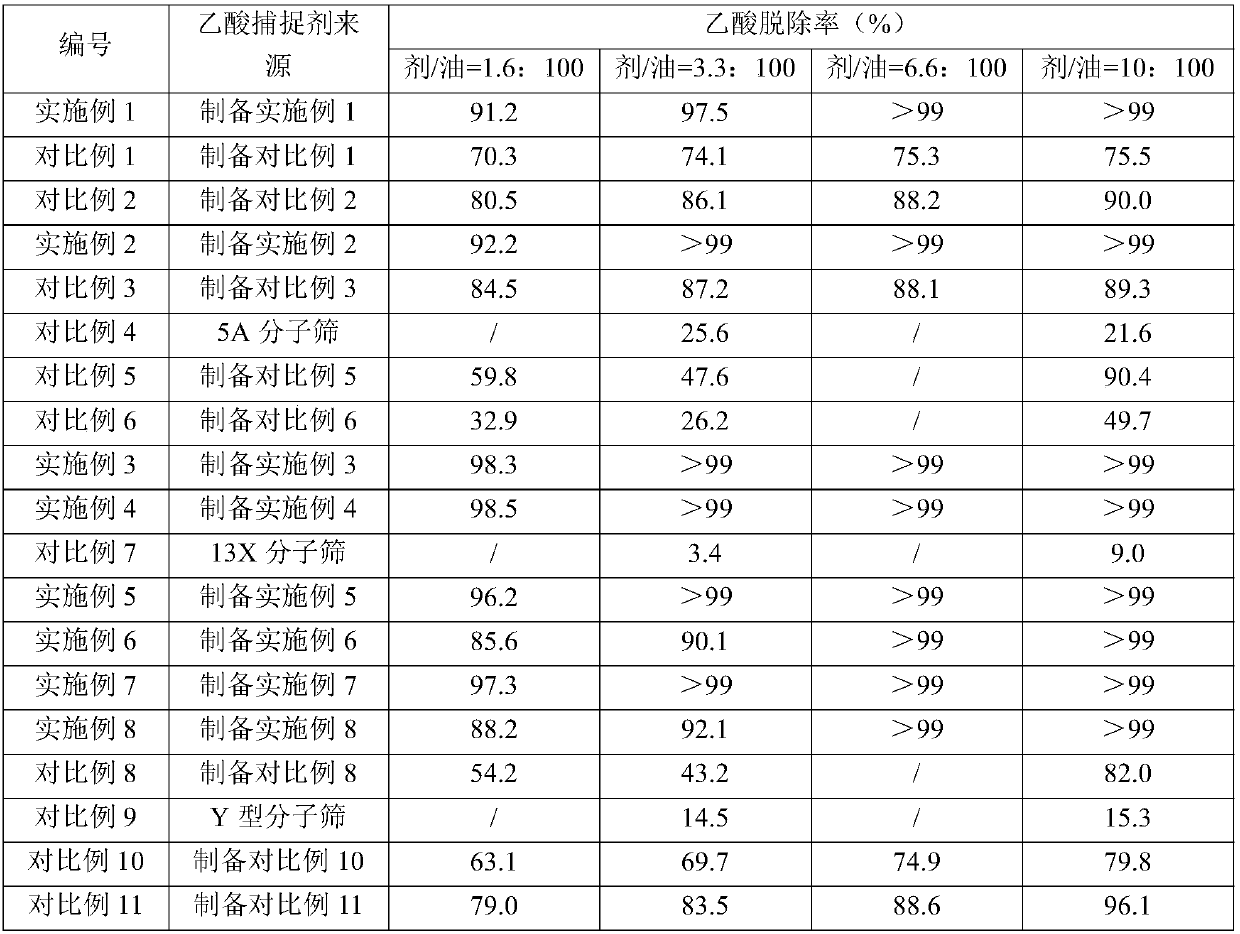
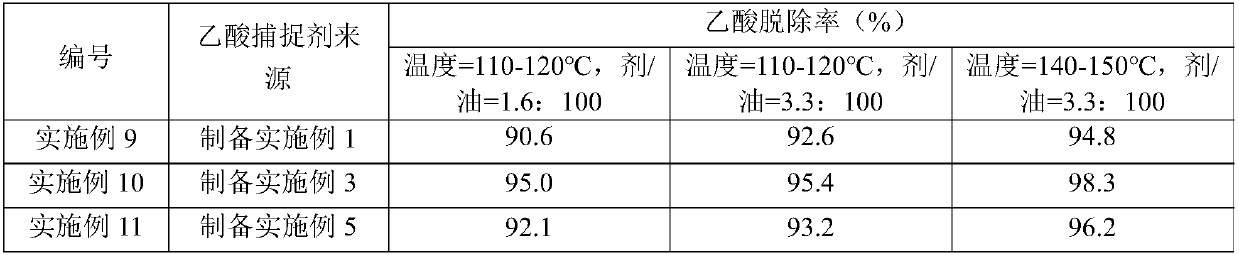
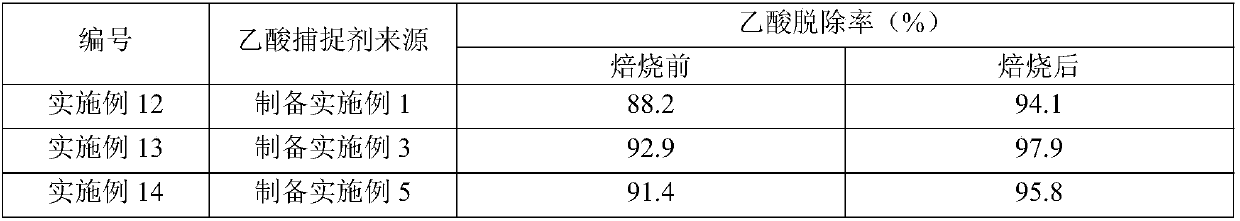
Examples
preparation Embodiment 1
[0132] 50kg Ca(OH) 2 , 10kg (based on the dry basis of molecular sieve) 5A molecular sieve (purchased from Henan Mingze Environmental Protection Technology Co., Ltd.), 30kg MgCO 3 And 5kg aluminum sol (purchased from Shandong Zhilida Chemical Co., Ltd., the alumina content is 12% by weight) is placed in the disc granulator (purchased from Shanghai Keruichi Chemical Equipment Technology Co., Ltd.), gradually spraying deionized water to make the material gradually into a ball. The obtained spheres (3-6 mm in diameter) were dried at 120° C. for 8 h, and then calcined at 600° C. in an air atmosphere for 4 h to obtain an acetic acid scavenger whose composition is listed in Table 1.
preparation Embodiment 2
[0138] The acetic acid scavenger is prepared in the same manner as in Preparation Example 1, except that Ca(OH) 2 The dosage is 77kg.
preparation Embodiment 3
[0148] With 60kg CaO, 8kg (based on the dry basis of molecular sieve) 13X molecular sieve (purchased from Langfang Pengcai Fine Chemical Co., Ltd.), 10kg Mg(OH) 2 And 5kg silica sol (purchased from Zhejiang Yuda Chemical Co., Ltd., silicon dioxide content is 25% by weight) is placed in the disc granulator (purchased from Shanghai Keruichi Chemical Equipment Technology Co., Ltd.), gradually spraying deionized water to make the material gradually into a ball. The obtained spheres (3-6 mm in diameter) were dried at 140° C. for 6 h, and then calcined at 520° C. in an air atmosphere for 6 h to obtain an acetic acid scavenger. The composition of the acetic acid scavenger is listed in Table 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thermal resistance | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com