Indolo-pyrroloquinoline compounds and synthesis method thereof
A technology of indolopyrrole and synthesis method, applied in directions such as organic chemistry, can solve the problems of small expansion range of substrates, harsh reaction conditions, difficult to obtain raw materials, etc., and achieves large expansion range of substrates and high diastereoselectivity. , the effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
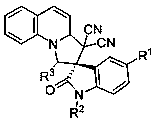
[0024] Example 1, preparation of 3',3'-dicyano-2-oxo-3',3a'-dihydro-1'H-spiro[indoline-3,2'-pyrrolo with the following structural formula [1,2-a]quinoline]-1'-ethyl carboxylate, the reaction formula is as follows:
[0025]
[0026] The preparation method is as follows:
[0027] In a 50mL round bottom flask, add 0.2950g (1.0mmol) quinoline quaternary ammonium salt, 0.1950g (1.0mmol) isatin malononitrile, 0.1517g (1.5mmol) Et 3 N was used as a basic catalyst and 10 mL of dry acetonitrile was used as a solvent, stirred at room temperature for 12 hours, and the reaction progress was detected by thin-layer chromatography.
[0028] After the reaction was completed, the solvent was concentrated and evaporated on a rotary evaporator, and separated by column chromatography (mixing ethyl acetate and petroleum ether in a volume ratio of 1:3, and used as a developing solvent for column chromatography separation) to obtain pure 3' , 3'-dicyano-2-oxo-3',3a'-dihydro-1'H-spiro[indoline-3...
Embodiment 2
[0031] Example 2, preparation of 5-chloro-3', 3'-dicyano-2-oxo-3', 3a'-dihydro-1'H-spiro[indoline-3,2 Ethyl '-pyrrolo[1,2-a]quinoline-1'-carboxylate:
[0032]
[0033] In a 50mL round bottom flask, add 0.2950g (1.0mmol) quinoline quaternary ammonium salt, 0.1950g (1.0mmol) isatin malononitrile, 0.1517g (1.5mmol) Et 3 N was used as a basic catalyst and 10 mL of dry acetonitrile was used as a solvent, stirred at room temperature for 12 hours, and the reaction progress was detected by thin-layer chromatography.
[0034] After the reaction was completed, the solvent was concentrated and evaporated on a rotary evaporator, and separated by column chromatography (mixing ethyl acetate and sherwood oil with a volume ratio of 1:3, and performing column chromatography separation as a developer), in Example 1 , the isatin malononitrile used is replaced with 5-chloroisatin malononitrile in equimolar amounts, and other steps are the same as in Example 1 to obtain 5-chloro-3', 3'-dicyano...
Embodiment 3
[0037] Example 3, preparation of 3',3'-dicyano-5-methyl-2-oxo-3',3a'-dihydro-1'H-spiro[indoline-3, 2'-Pyrrolo[1,2-a]quinoline]-1'-carboxylic acid ethyl ester:
[0038]
[0039] In Example 1, the isatin malononitrile used is replaced with an equimolar amount of 5-methyl isatin malononitrile, and the other steps are the same as in Example 1 to obtain 3', 3'-dicyano-5-methanol yl-2-oxo-3',3a'-dihydro-1'H-spiro[indoline-3,2'-pyrrolo[1,2-a]quinoline]-1'-carboxylic acid Ethyl ester, its isolated yield is 87%, and structural characterization data is as follows:
[0040] 1 H NMR (400 MHz, DMSO- d 6 ) δ: major isomer: 11.34 (s, 1H, NH), 7.44 (m,1H, ArH), 7.35 (d, J = 8.0 Hz, 1H, ArH), 7.12-7.06 (m, 2H, ArH), 6.96-6.87(m, 2H, ArH), 6.78-6.71 (m, 1H, ArH), 6.50 (d, J = 8.0 Hz, 1H, ArH), 6.06-6.05 (m, 1H, ArH), 5.92-5.86 (m, 1H, CH), 5.16 (s, 1H, CH), 4.19-4.13 (m, 1H, CH) , 3.98-3.92 (m, 1H, CH), 2.30 (s, 3H, CH 3 ), 1.06 (t, J = 7.2 Hz, 3H, CH 3 ); minor isomer: 11.37 (s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com