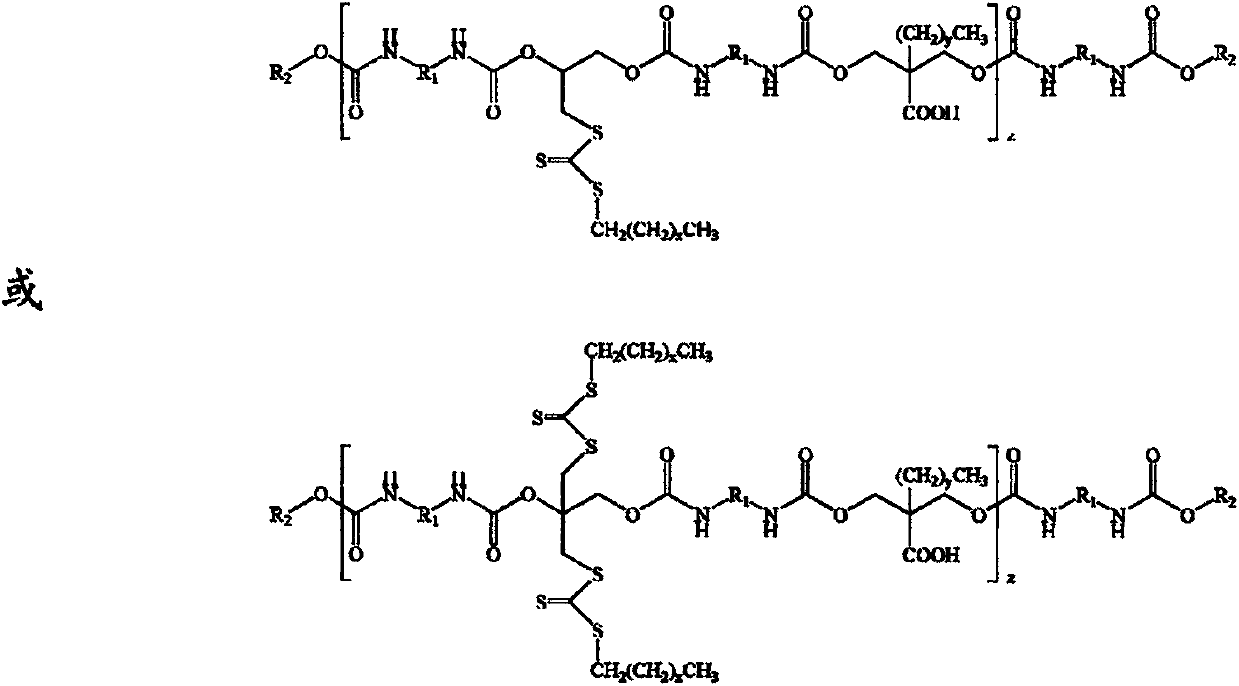
Preparation method of polyurethane RAFT (reversible addition fragmentation chain transfer) reagent
A technology of polyurethane and reagents, which is applied in the field of preparation of polyurethane RAFT reagents, can solve the problems of complex process, low yield, and inability to produce on a large scale, and achieve the effect of simple process and convenient purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
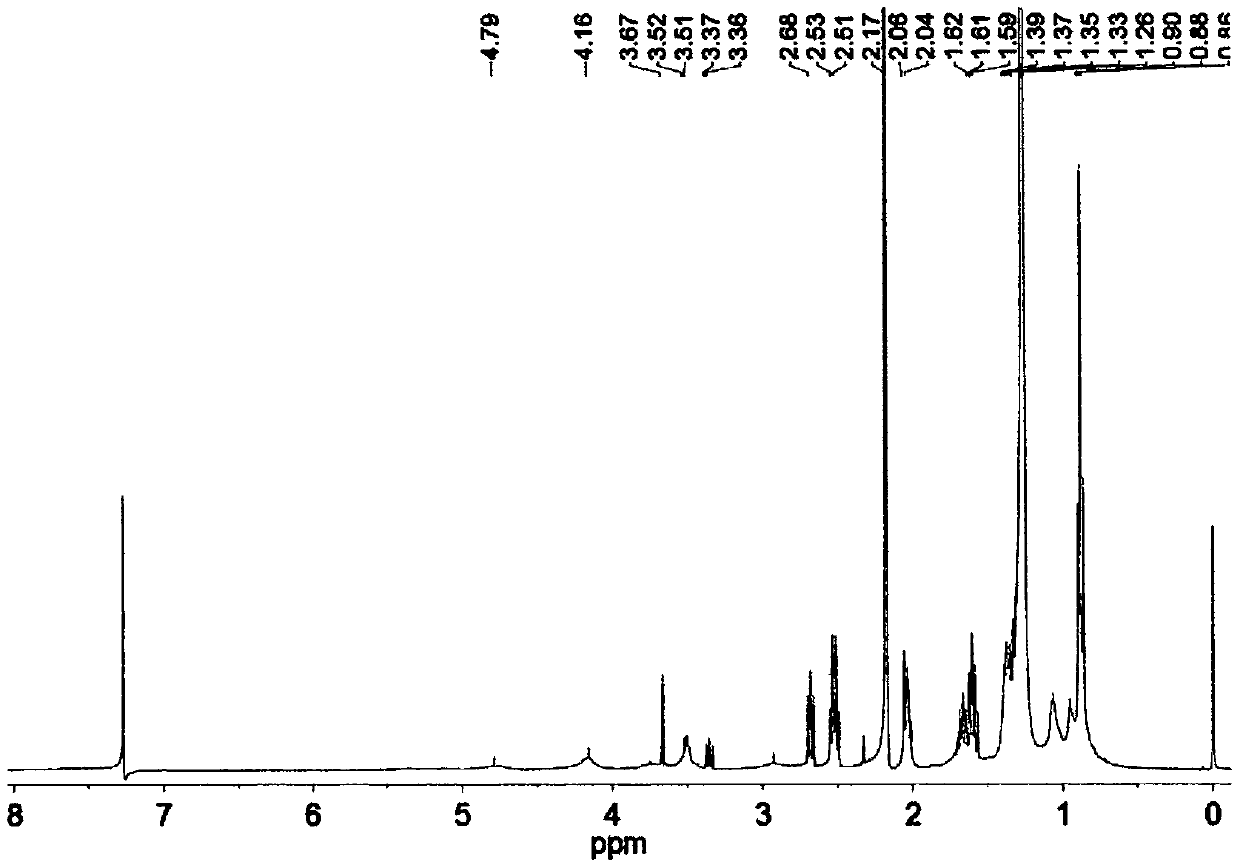
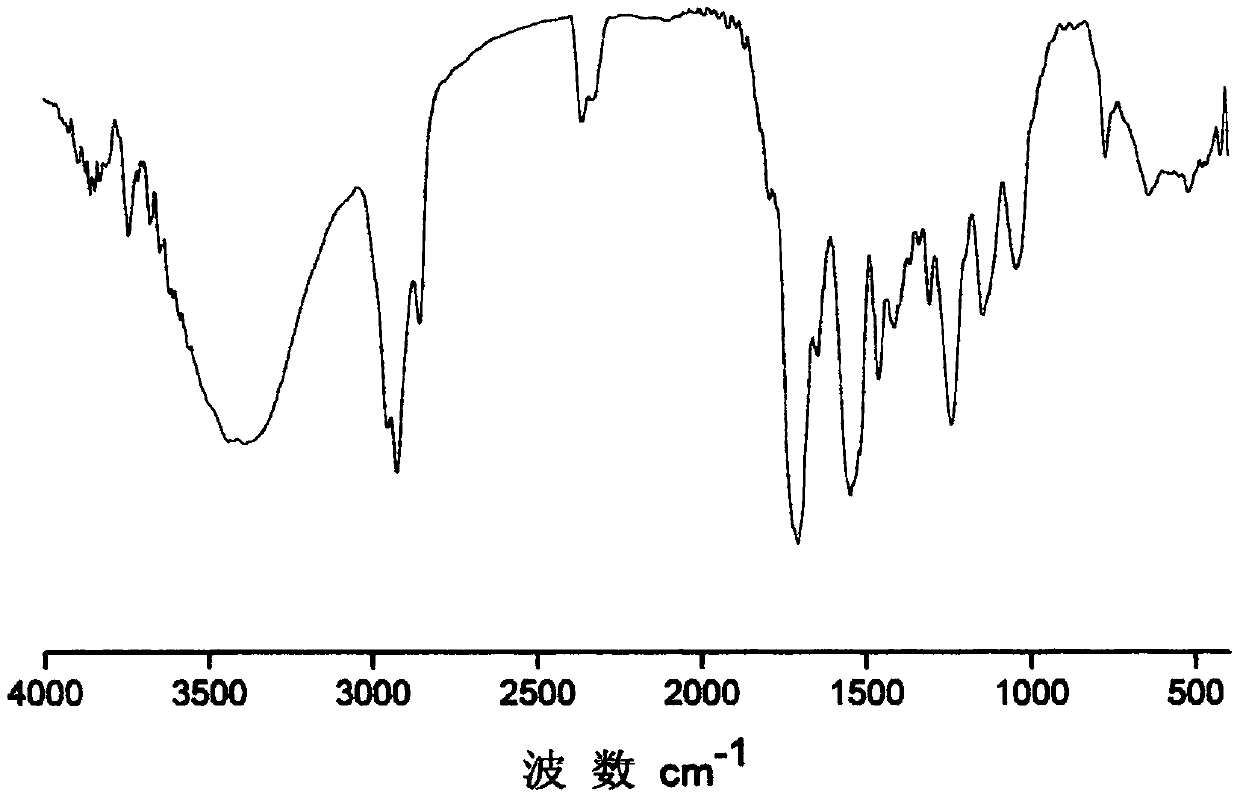
[0028] Step 1, add 8.91g (0.04mol) isophorone diisocyanate, 3.08g (0.028mol) 3-chloro-1,2-propanediol, 1.48g (0.01mol) dimethylol butyric acid, 0.04g (6.33×10 -5 mol) dibutyltin dilaurate, 5.00 mL of anhydrous acetone, stirred and reacted at 80°C for 6 hours, added 0.52 g (0.004 mol) of n-octanol and reacted for 2 hours, the reaction was completed, and set aside.
[0029] Step 2, the reaction kettle is placed on an ice bath, 5.67g (0.028mol) of dodecyl mercaptan is added thereto, under magnetic stirring, 3.73g of 30wt% NaOH aqueous solution is slowly added dropwise to the flask, and after 15min, under stirring Add acetone 5.00mL, after fully dissolved, add 2.13g (0.028mol) CS 2 , continue to stir the reaction for 1h. Then add the polyurethane prepared in step 1, stir and react at 30°C for 12 hours, the product is precipitated in 250mL volume ratio of 1:1 methanol / water, after the precipitate is dried at room temperature, it is placed in a vacuum drying oven at 90°C and dried...
Embodiment 2
[0031] Step 1. Add 8.91g (0.04mol) isophorone diisocyanate, 7.33g (0.028mol) 2,2-dibromomethyl-1,3-propanediol, 1.48g (0.01mol) dihydroxy Methylbutyric acid, 0.04g (6.33×10 -5 mol) dibutyltin dilaurate, 5.00 mL of anhydrous acetone, stirred and reacted at 80°C for 6 hours, added 0.52 g (0.004 mol) of n-octanol and reacted for 2 hours, the reaction was completed, and set aside.
[0032] Step 2, the reaction kettle is placed on an ice bath, 5.67g (0.028mol) of dodecyl mercaptan is added thereto, under magnetic stirring, 3.73g of 30wt% NaOH aqueous solution is slowly added dropwise to the flask, and after 15min, under stirring Add acetone 5.00mL, after fully dissolved, add 2.13g (0.028mol) CS 2 , continue to stir the reaction for 1h. Then add the polyurethane prepared in step 1, stir and react at 30°C for 12 hours, the product is precipitated in 250mL volume ratio of 1:1 methanol / water, after the precipitate is dried at room temperature, it is placed in a vacuum drying oven at ...
Embodiment 3
[0034] Step 1. Add 6.73g (0.04mol) hexamethylene diisocyanate, 3.08g (0.028mol) 3-chloro-1,2-propanediol, 1.07g (0.008mol) dimethylol propionic acid, 0.05g (7.92×10 -5 mol) dibutyltin dilaurate, 5.00mL of anhydrous acetone, stirred and reacted at 85°C for 8h, added 1.04g (0.008mol) of n-octanol and reacted for 2h, the reaction was completed and set aside.
[0035] Step 2, the reaction kettle is placed on an ice bath, 5.67g (0.028mol) of dodecyl mercaptan is added thereto, and under magnetic stirring, 3.73g of 30wt% NaOH aqueous solution is slowly added dropwise to the flask, and after 15min, under stirring Add acetone 5.00mL, after fully dissolved, add 2.13g (0.028mol) CS 2, continue to stir the reaction for 1h. Then add the polyurethane prepared in step 1, stir and react at 50°C for 6 hours, the product is precipitated in 250mL volume ratio of 2:1 methanol / water, after the precipitate is dried at room temperature, it is placed in a vacuum drying oven at 90°C and dried overn...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com