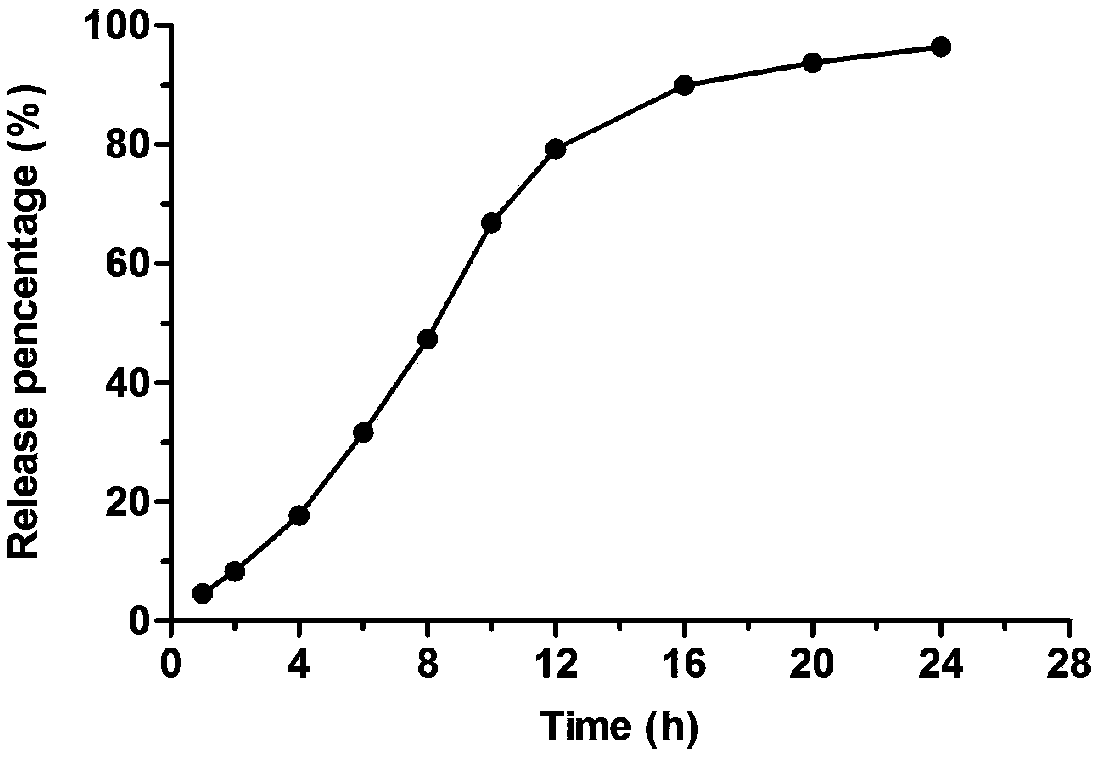
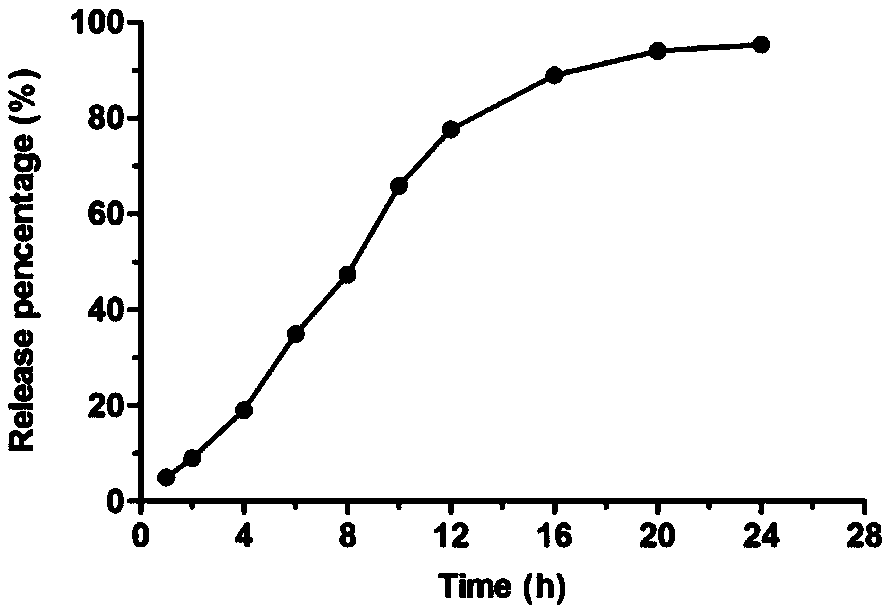
Glipizide lipid nanoparticle solid preparation
A technology for glipizide lipid and solid preparation, which is applied in the field of medicine and achieves the effects of high encapsulation efficiency, narrow particle size distribution and improved quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Example 1 Preparation of Glipizide Lipid Nanoparticle Tablets
[0046] (1) Preparation of Glipizide Solid Lipid Nanoparticles
[0047] The prescription is as follows:
[0048] Glipizide 10 g
[0049] Trilaurin 140 g
[0050] Tween 80 80 g
[0051] Chitosan 28 g
[0052] The preparation method is as follows:
[0053] (a) Add 140 g of trilaurin to 500 mL of a mixed solution of ethanol and acetone with a volume ratio of 1:1, heat in a constant temperature water bath at 55°C, stir to dissolve, then add 10 g of glipizide, and stir Make it dissolve, constitute the organic phase, and set aside;
[0054] (b) Add 80 g of Tween (80) and 28 g of chitosan into 500 mL of purified water, heat in a constant temperature water bath at 55 °C, stir to dissolve, and form a water phase for later use;
[0055] (c) Under the condition of stirring at 800 rpm, slowly add the organic phase to the water phase, keep the temperature at 55 °C, and stir for 30 minutes;
[0056] (d) Concentrat...
Embodiment 2
[0072] Example 2 Preparation of Glipizide Lipid Nanoparticle Tablets
[0073] (1) Preparation of Glipizide Solid Lipid Nanoparticles
[0074] The prescription is as follows:
[0075] Glipizide 10 g
[0076] Trilaurin 120 g
[0077] Tween 80 68 g
[0078] Chitosan 32 g
[0079] The preparation method is as follows:
[0080] (a) Add 120 g of trilaurin to 500 mL of a mixed solution of ethanol and acetone with a volume ratio of 1:1, heat in a constant temperature water bath at 55°C, stir to dissolve, then add 10 g of glipizide, and stir Make it dissolve, constitute the organic phase, and set aside;
[0081] (b) Add 68 g of Tween (80) and 32 g of chitosan into 500 mL of purified water, heat in a constant temperature water bath at 55 °C, stir to dissolve, and form a water phase for later use;
[0082] (c) Under the condition of stirring at 800 rpm, slowly add the organic phase to the water phase, keep the temperature at 55 °C, and stir for 30 minutes;
[0083] (d) Concentrat...
Embodiment 3
[0099] Example 3 Preparation of Glipizide Lipid Nanoparticle Capsules
[0100] (1) Preparation of Glipizide Solid Lipid Nanoparticles
[0101] The prescription is as follows:
[0102] Glipizide 10 g
[0103] Trilaurin 160 g
[0104] Tween 80 90 g
[0105] Chitosan 20 g
[0106] The preparation method is as follows:
[0107] (a) Add 160 g of glycerol trilaurate into 500 mL of a mixed solution of ethanol and acetone with a volume ratio of 1:1, heat in a constant temperature water bath at 55°C, stir to dissolve, then add 10 g of glipizide, and stir Make it dissolve, constitute the organic phase, and set aside;
[0108] (b) Add 90 g of Tween (80) and 20 g of chitosan into 500 mL of purified water, heat in a constant temperature water bath at 55 °C, stir to dissolve, and form a water phase for later use;
[0109] (c) Under the condition of stirring at 800 rpm, slowly add the organic phase to the water phase, keep the temperature at 55 °C, and stir for 30 minutes;
[0110] (...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com