Chalcone-benzimidazole salt compounds and preparation thereof
A technology of benzimidazole and chalcone, applied in the field of chalcone-benzimidazole salt compound and its preparation, can solve the problems of acquired drug resistance and complex chemical structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
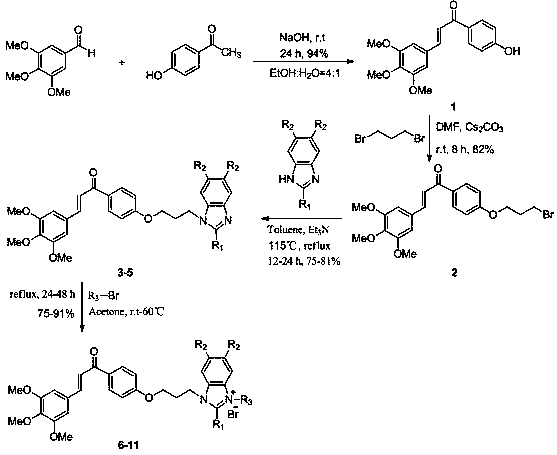
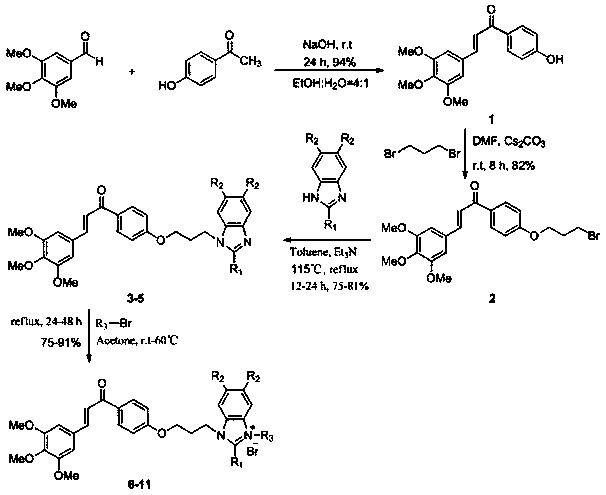
[0021] The preparation method specifically includes:
[0022] A, the preparation of chalcone structure intermediate 1:
[0023] In a 100 mL round bottom flask, dissolve 3,4,5-trimethoxybenzaldehyde (7.00 mmol) in ethanol: water = 4:1 (35 mL) solvent at room temperature, add sodium hydroxide (14 mmol), stirred for 15 minutes, added 4-hydroxyacetophenone (8.4 mmol), stirred at room temperature for 24 hours, TLC thin-layer chromatography detected the reaction until the reaction was complete, extracted with ethyl acetate, washed with saturated brine, combined organic layers, Drying over anhydrous magnesium sulfate, the organic layer was evaporated to dryness under reduced pressure, and the concentrated solution was subjected to silica gel column chromatography (petroleum ether: ethyl acetate = 1:1) to obtain compound 1 as a yellow solid (yield: 94%).
[0024] B, the preparation of bromoalkane chalcone intermediate 2:
[0025] In a 100 mL round-bottom flask, dissolve compound 1 (...
Embodiment 1
[0032] Preparation of compound 6: see the above preparation methods A, B, C, D:
[0033]
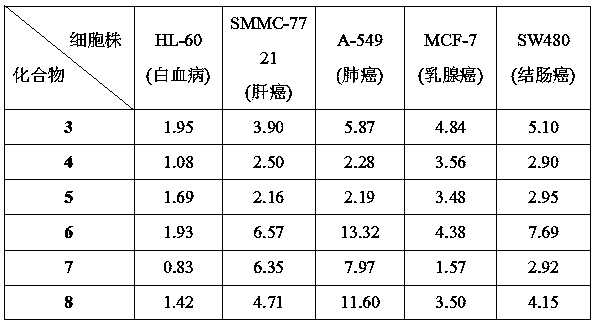
[0034] Compound 6: Formula C 37 h 38 Br 2 N 2 o 5。 Yield 79%, yellow solid, melting point: 122.4-123.3 ℃; IR(KBr) ν max (cm -1 ): 3450, 2361, 2341, 2027, 1655, 1603, 1460, 1160, 1068, 952,861, 547. 1 H NMR (400 MHz, DMSO), δ (ppm): 9.76 (1H, s), 8.16 (2H, d, J = 8.7Hz), 7.90 (2H, d, J = 15.2 Hz), 7.73 (2H, d, J = 5.8 Hz), 7.67 (1H, d, J =15.5 Hz), 7.40 (2H, t, J = 8.3 Hz), 7.23 (2H, s), 6.97 (2H, d, J = 8.9 Hz),5.77 (2H, s), 4.72 (2H, t, J = 13.3 Hz), 4.20 (2H, t, J = 11.2 Hz), 3.88 (6H, s), 3.72 (3H, s), 2.51 (6H, d, J = 1.7 Hz), 2.35 (3H, d, J =6.2 Hz). 13C NMR(100 MHz, DMSO), δ (ppm): 187.74, 162.45, 153.58, 144.20, 142.99, 142.36,140.11,, 137.47, 137.06, 133.74, 133.25, 133.17, 131.39, 131.37, 131.15,130.93, 130.81, 130.66 , 130.27, 130.19, 130.10, 128.91, 124.34, 124.35,123.34, 121.61, 114.79, 113.60, 106.97, 65.43, 60.62, 56.65, 50.89,50.74, 28.63, ...
Embodiment 2
[0036] Preparation of compound 7: see the above preparation methods A, B, C, D:
[0037]
[0038] Compound 7: Formula C 41 h 41 BrN 2 o 5, Yield 89%, yellow solid, melting point: 166.2-166.9 ℃; IR(KBr) ν max (cm -1 ): 3450, 2344, 2027, 1657, 1605, 1564, 1461, 1313, 1272, 1218, 1160, 1068, 952, 861, 548. 1 H NMR (400 MHz, DMSO), δ (ppm): 9.93 (1H, s), 8.14 (1H, d, J = 8.2Hz), 8.06 (1H, s), 7.89 (5H,m), 7.68 (1H, d, J = 15.4Hz), 7.56 (3H, s), 7.23 (2H, s), 6.95 (2H, d, J = 8.2 Hz), 5.89 (2H, s), 4.71(2H, s), 4.71 (2H, s), 4.22 (2H, s), 3.88 (6H, s), 3.73 (3H, s), 2.51 (3H ,s), 2.34 (6H, d, J = 9.5 Hz). 13 C NMR (100 MHz, DMSO), δ (ppm): 187.74,162.45, 153.59, 144.19, 141.98, 140.14, 136.90, 133.17, 131.96, 131.35,131.37, 130.81, 130.42, 129.99, 129.99, 129.24, 128.33, 128.14, HRMS (ESI-TOF) m / z Calcd for C 41 h 41 BrN 2 o 5 [M-Br] + 641.3015, found 641.3008.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com