Synthesis method and application of antibacterial medicine tri-carbon chain methylpiperidine urolithin B and hydrochloride thereof
A technology of three-carbon chain methylpiperidine and antibacterial drugs, which is applied in the field of synthetic antibacterial drugs, can solve problems such as difficult to carry out smoothly and poor antibacterial activity tests, and achieve the effect of increasing sources
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 3
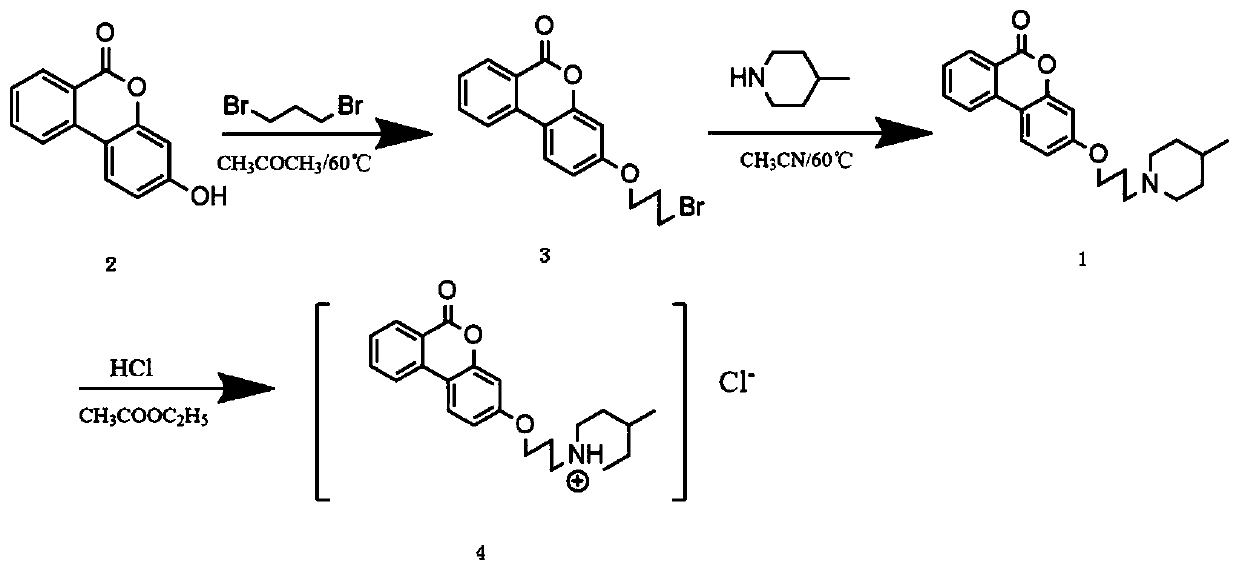
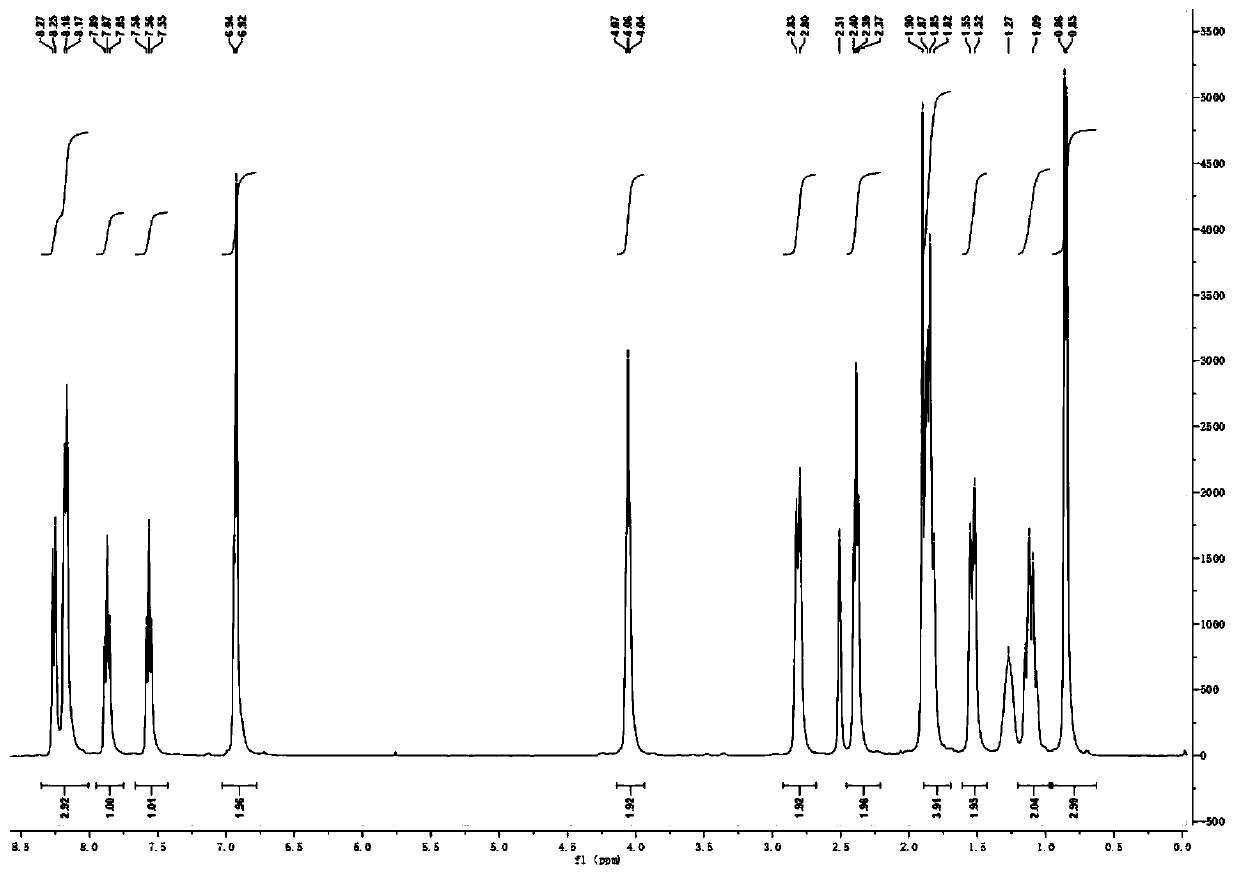
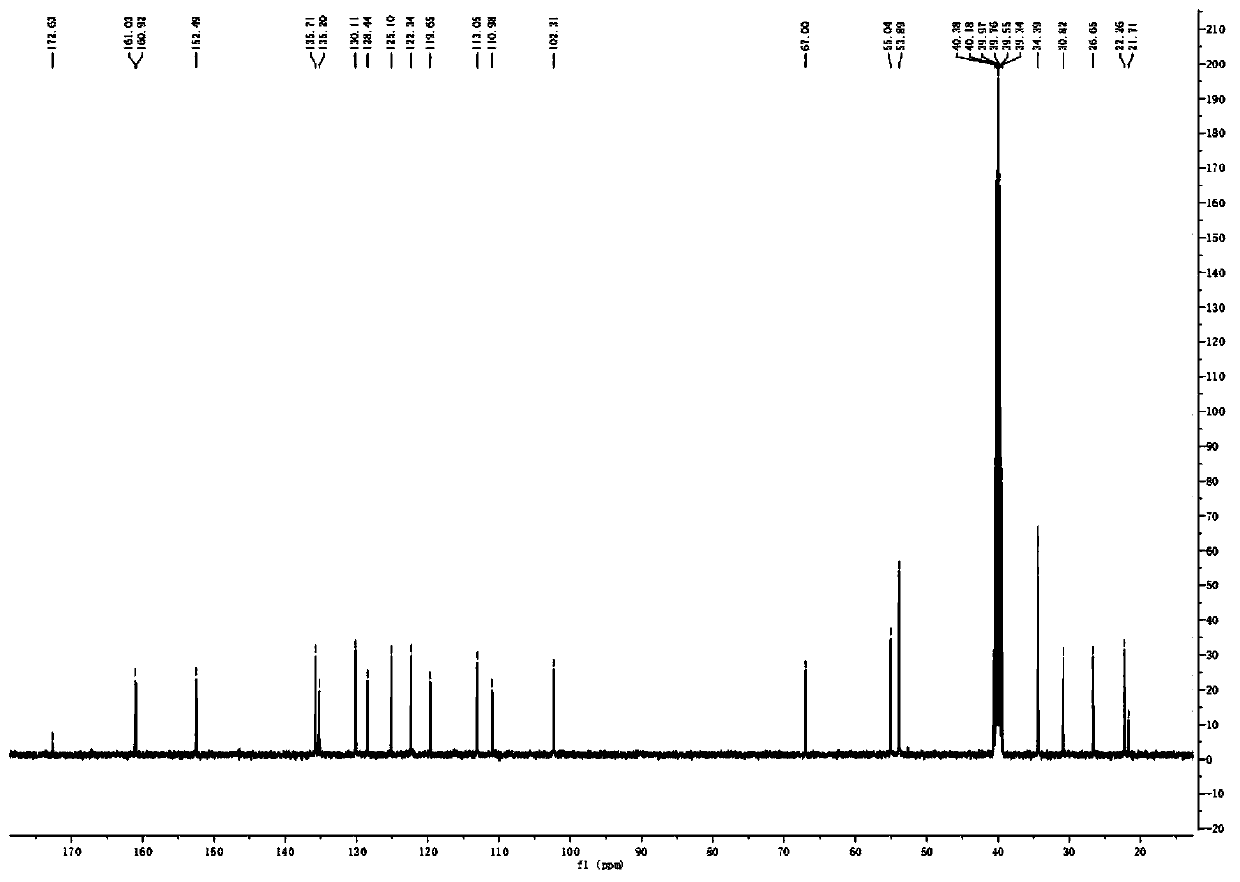
[0035] The synthetic method of embodiment 1 three-carbon chain methyl piperidine urolithin B hydrochloride
[0036] (1) Synthesis of bromopropanated urolithin B (3):
[0037] Take the reactant urolithin B (2), 1,3-dibromopropane, and potassium carbonate in a molar ratio of 1:4:4, and put the total reactant (g) and acetone (ml) in a round bottom flask at a ratio of 1:2 Reflux at 60°C for 2 hours, check whether the reaction is complete by thin-layer chromatography, the inspection standard is the disappearance of light blue urolithin B fluorescent spots, after the reaction is complete, spin the acetone, pour the solid powder into a beaker and add petroleum ether, stir, and use a Buchner funnel Remove excess 1,3-dibromopropane by suction filtration, pour the obtained solid into a beaker and add water to stir, then use a Buchner funnel to suction filter to obtain a filter cake, and dry the filter cake to obtain a crude sample of bromopropanated urolithin B.
[0038] (2) Synthesis ...
Embodiment 2 3
[0053] The antibacterial application of embodiment 2 three-carbon chain methyl piperidine urolithin B hydrochloride
[0054] (1) strain
[0055] Staphylococcus aureus ATCC25923, Escherichia coli ATCC25922, Staphylococcus epidermidis AB208188, Shigella flexneri BNCC108831, Klebsiella pneumoniae RM3017, all from the School of Life Sciences, Wuhan University; Escherichia coli CMCC44102, Listeria monocytogenes Bacteria CMCC54002 and Salmonella typhimurium CMCC50115 were purchased from Beijing Putian Biotechnology Co., Ltd.
[0056] (2) Bacterial suspension preparation
[0057] Take the above-mentioned glycerol-frozen bacteria and inoculate them on the nutrient agar plate by streaking, culture at 37°C for 18h to 24h, pick a single colony and inoculate them into the nutrient broth, culture at 37°C until the logarithmic growth phase, centrifuge at 5000rpm for 6min, and collect the bacteria , washed twice with phosphate buffered saline (PBS) and then resuspended to adjust the bacter...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com