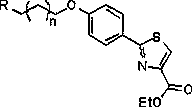
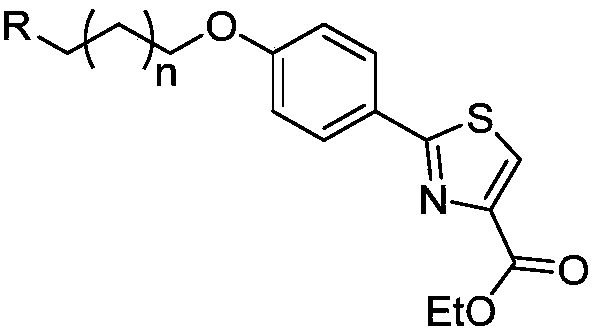
Preparation method and application of ethyl 2-(4-hydroxyphenyl)thiazole-4-carboxylate derivatives
A technology of ethyl carboxylate and hydroxyphenyl, which is applied in the field of preparation of ethyl 2-thiazole-4-carboxylate derivatives, can solve problems such as toxic side effects and failure to prevent the degeneration and death of central cholinergic neurons, and achieve Convenient post-processing, simple and safe synthesis method, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
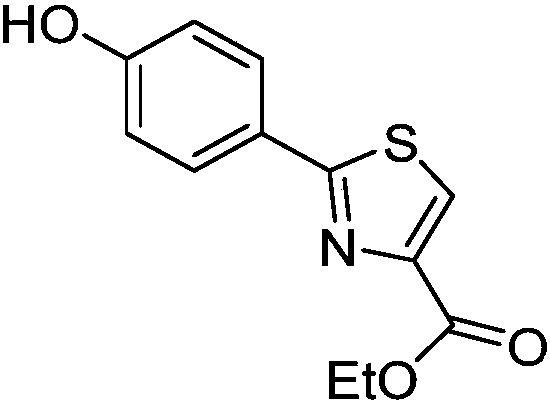
[0025] (1) Synthesis of ethyl 2-(4-hydroxyphenyl)thiazole-4-carboxylate (3)
[0026]
[0027] Take 3.93g of p-hydroxythiobenzamide in a round bottom flask, add 50.00mL of absolute ethanol and 5.00mL of ethyl 3-bromopyruvate with a mass fraction of 80%, heat at 80°C for 4h; cool to room temperature after the reaction , add 100mL distilled water, precipitate solid, suction filter, take solid, wash with water, obtain compound 2-(4-hydroxyphenyl) thiazole-4-carboxylate ethyl ester light yellow powder after vacuum drying, yield rate is 88.56%. 1 H NMR (500MHz,DMSO)δ10.11(s,1H),8.44(s,1H),7.85–7.75(m,2H),6.95–6.86(m,2H),4.33(q,J=7.1Hz, 2H), 1.33(t, J=7.1Hz, 3H).
[0028] (2) Synthesis of ethyl 2-(4-(3-bromopropoxy)phenyl)thiazole-4-carboxylate (4a)
[0029]
[0030] Take 0.50 g of the compound obtained from the reaction in step (1) in a round bottom flask, add 0.55 g of anhydrous potassium carbonate, 1.13 mL of 1,3-dibromopropane and 6.00 mL of dimethylformamide DMF. Heatin...
Embodiment 2
[0035] The difference between embodiment 2 and embodiment 1 is:
[0036] (3) Synthesis of ethyl 2-(4-(3-(propylamino)propoxy)phenyl)thiazole-4-carboxylate (5a-2)
[0037]
[0038] The preparation method is the same as in Example 1, wherein the amines are replaced by di-n-propylamine. A yellow liquid, compound 5a-2, was obtained with a yield of 84.34%. 1 H NMR (500MHz, CDCl 3 )δ8.09(s,1H),7.94(d,J=8.8Hz,2H),6.94(d,J=8.8Hz,2H),4.44(q,J=7.1Hz,2H),4.09(t, J=6.1Hz, 2H), 2.79(s, 2H), 2.56(d, J=6.8Hz, 4H), 2.14–2.00(m, 2H), 1.58(dd, J=15.0, 7.4Hz, 4H), 1.43(t,J=7.1Hz,3H),0.91(t,J=7.4Hz,6H).; 13 C NMR (126MHz, CDCl 3 )δ168.74,161.55,160.91,147.82,128.54,126.27,125.76,114.73,66.06,61.45,55.67,53.44,50.46,19.24,14.38,11.75.HRMS:(ESI,m / z):[M+H] + calcd for C 21 h 30 N 2 o 3 S 391.2050, found 391.2053.
Embodiment 3
[0040] The difference between embodiment 3 and embodiment 1 is:
[0041] (3) Synthesis of ethyl 2-(4-(3-(dimethylamino)propoxy)phenyl)thiazole-4-carboxylate (5a-3)
[0042]
[0043] The preparation method is the same as in Example 1, wherein the amines are replaced by diethylamine. A light yellow powder was obtained, namely compound 5a-3, with a yield of 90.27%. m.p.64-66℃.; 1 H NMR (500MHz, CDCl 3 )δ8.09(s,1H),7.94(d,J=8.2Hz,2H),6.95(d,J=8.3Hz,2H),4.44(q,J=7.1Hz,2H),4.09(t, J=5.9Hz, 2H), 2.80–2.75(m, 2H), 2.71(dd, J=13.3, 6.4Hz, 4H), 2.13–2.01(m, 2H), 1.43(t, J=7.1Hz, 3H ),1.14(t,J=7.0Hz,6H).; 13 C NMR (126MHz, CDCl 3 )δ168.74,161.55,160.91,147.82,128.53,126.26,125.76,114.75,66.17,61.46,49.26,46.91,26.16,14.38,10.93.HRMS:(ESI,m / z):[M+H] + calcd for C 19 h 26 N 2 o 3 S 363.1737,found 363.1740.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com