Preparation methods of rare earth layered hydroxide nanosheets and rare earth layered hydroxide nanosheet sol
A layered hydroxide and nanosheet technology, applied in the field of material science, can solve the problems of slow hydrolysis, poor dispersibility, poor stability, etc., and achieve the effects of simple preparation process, saving raw materials, and convenient operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
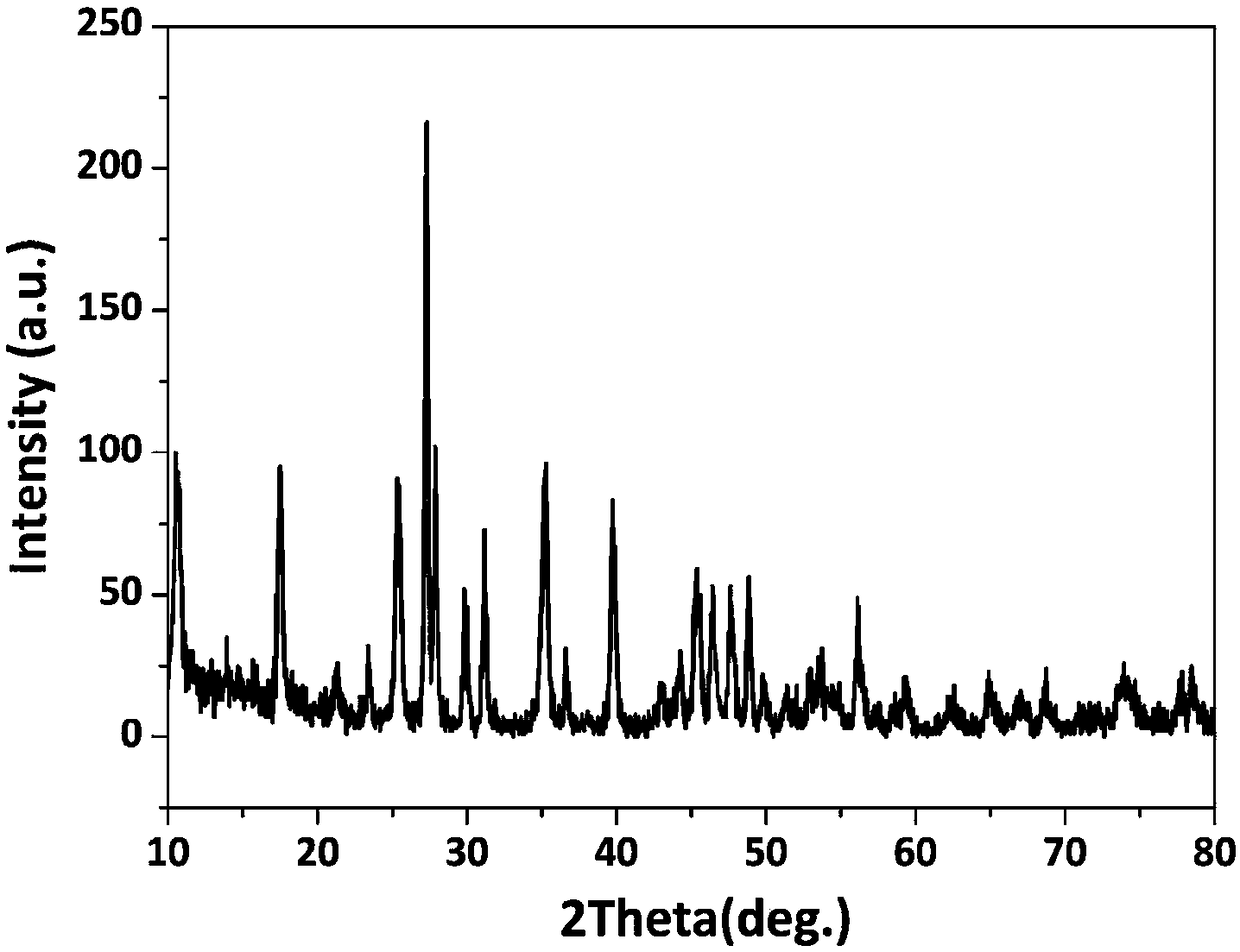
[0033] A kind of rare earth layered hydroxide La 2 (OH) 4 SO 4 ·nH 2 The preparation method of O nanosheet is characterized in that, comprises the following steps:
[0034] (1) Lanthanum nitrate hexahydrate (La(NO 3 ) 3 ·6H 2 O) Mix well in deionized water to prepare La 3+ Lanthanum nitrate (La(NO) with a concentration of 0.03mol / L 3 ) 3 ) solution;
[0035] (2) The amount added is in molar ratio, SO 4 2- : La 3+ =0.5, ammonium sulfate particles ((NH 4 ) 2 SO 4 ) dissolved in lanthanum nitrate (La(NO 3 ) 3 ) solution, dissolved for 10 minutes until completely clear to form a mixed solution;
[0036] (3) Add ammonia water to the mixed solution, adjust the pH of the mixed solution to 6, and continue to stir for 10 minutes to obtain a uniform suspension;
[0037] (4) React the homogeneous suspension, the reaction temperature is 4°C, the reaction time is 48h, and the product is obtained. The product composition is the rare earth layered hydroxide La containing in...
Embodiment 2
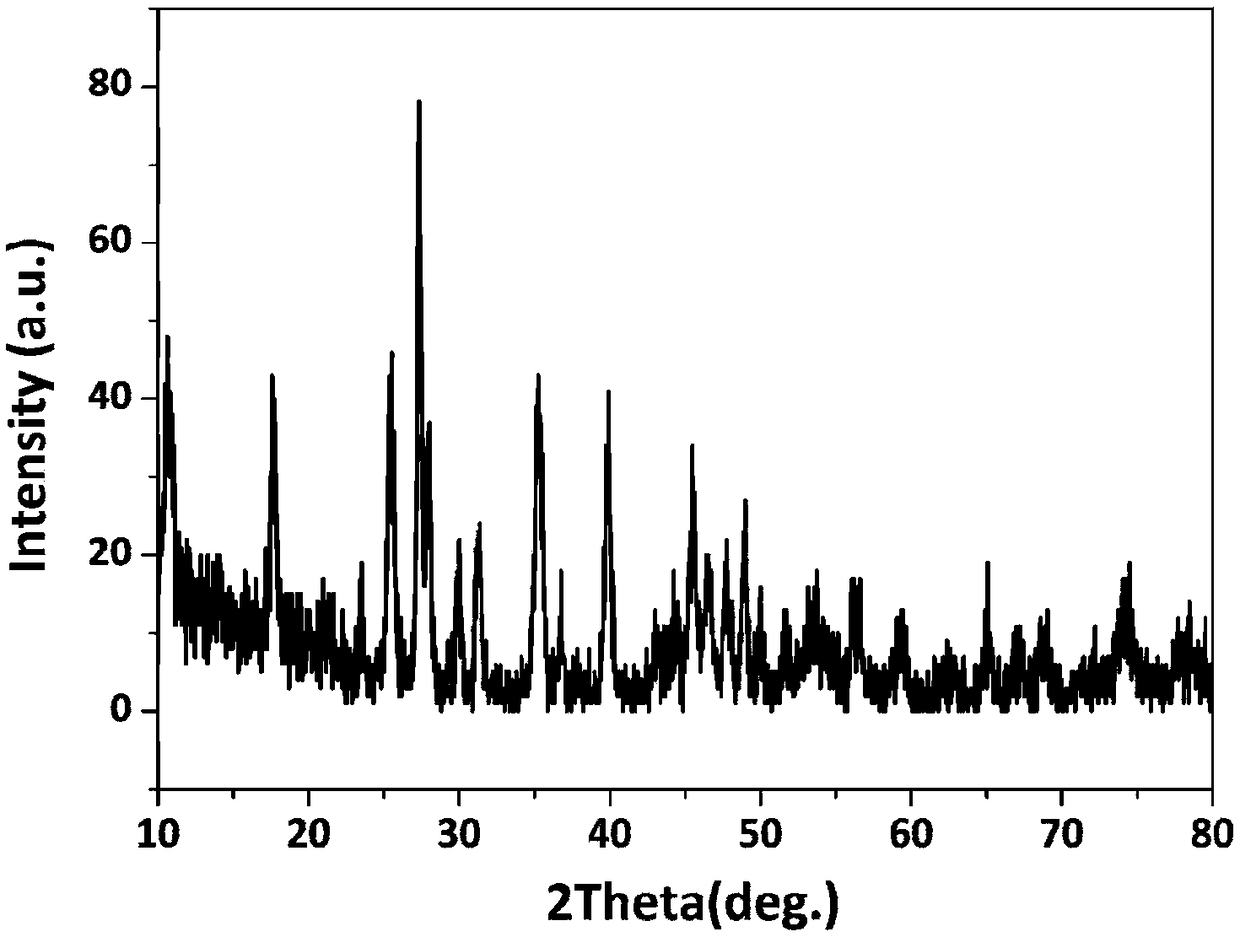
[0043] A kind of rare earth layered hydroxide La 2 (OH) 4 SO 4 ·nH 2 The preparation method of O nanosheet is characterized in that, comprises the following steps:
[0044] (1) Lanthanum nitrate hexahydrate (La(NO 3 ) 3 ·6H 2 O) Mix well in deionized water to prepare La 3+ Lanthanum nitrate (La(NO) with a concentration of 0.20mol / L 3 ) 3 ) solution;
[0045] (2) The amount added is in molar ratio, SO 4 2- : La 3+ =10, ammonium sulfate particles ((NH 4 ) 2 SO 4 ) dissolved in lanthanum nitrate (La(NO 3 ) 3 ) solution, dissolved for 20min until completely clear to form a mixed solution;
[0046] (3) Add ammonia water to the mixed solution, adjust the pH of the mixed solution to 10, and continue to stir for 30 minutes to obtain a uniform suspension;
[0047] (4) React the homogeneous suspension with a reaction temperature of 0°C and a reaction time of 1 h to obtain the product, which consists of rare earth layered hydroxide La containing incompletely reacted mot...
Embodiment 3
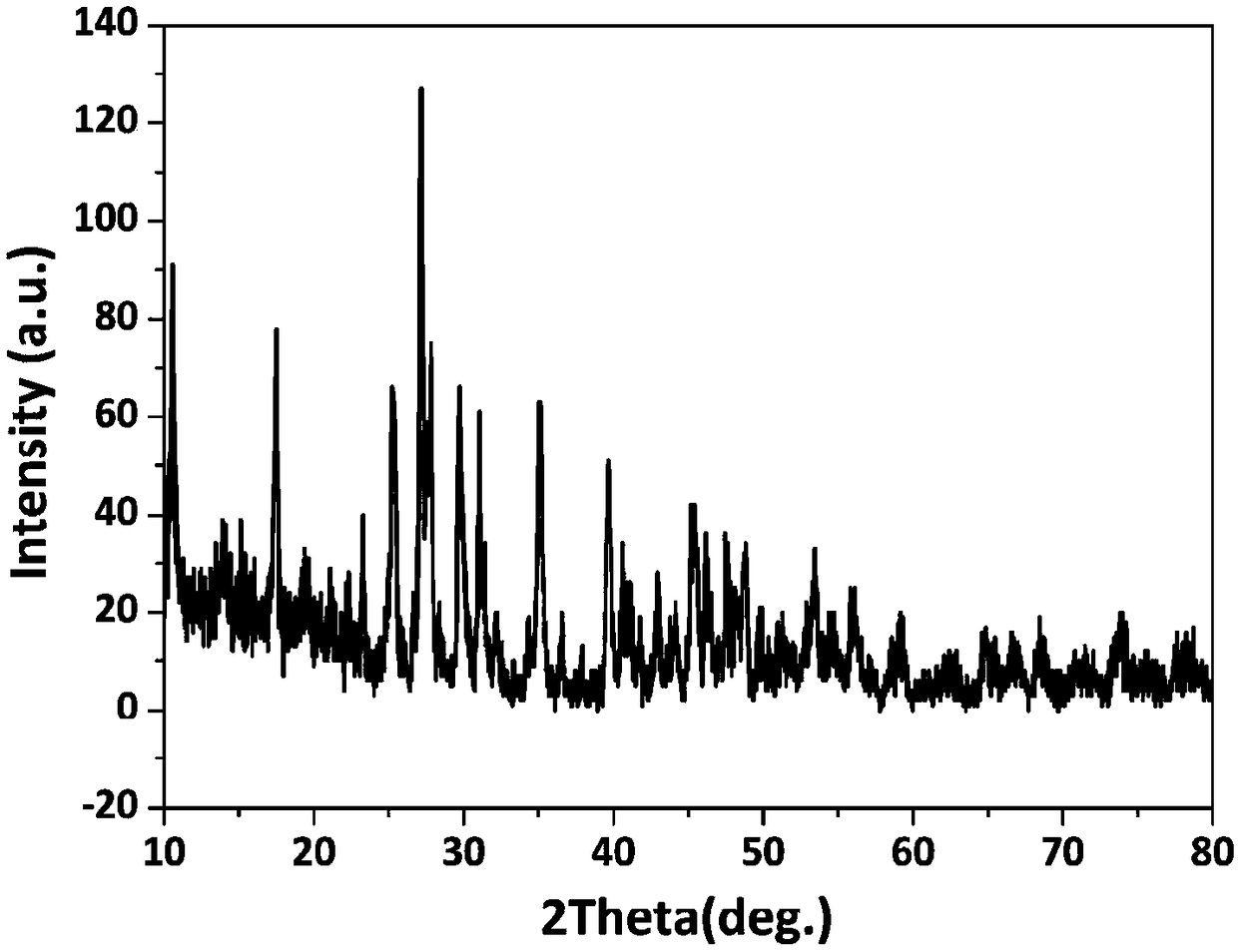
[0053] A kind of rare earth layered hydroxide La 2 (OH) 4 SO 4 ·nH 2 The preparation method of O nanosheet is characterized in that, comprises the following steps:
[0054] (1) Lanthanum nitrate hexahydrate (La(NO 3 ) 3 ·6H 2 O) Mix well in deionized water to prepare La 3+ Lanthanum nitrate (La(NO) with a concentration of 0.1mol / L 3 ) 3 ) solution;
[0055] (2) The amount added is in molar ratio, SO 4 2- : La 3+ =3, ammonium sulfate particles ((NH 4 ) 2 SO 4 ) dissolved in lanthanum nitrate (La(NO 3 ) 3 ) solution, dissolved for 14min until completely clear to form a mixed solution;
[0056] (3) Add ammonia water to the mixed solution, adjust the pH of the mixed solution to 9, and continue to stir for 20 minutes to obtain a uniform suspension;
[0057] (4) React the homogeneous suspension with a reaction temperature of -4°C and a reaction time of 24 hours to obtain the product, which consists of rare earth layered hydroxide La containing incompletely reacted ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com