CYP76B74 protein participating in biological synthesis of alkannin as well as encoding gene and application thereof
A technology of shikonin and coding, which is applied in the fields of plant gene improvement, application, genetic engineering, etc., and can solve problems such as unobtained amino acid sequence of hydroquinone
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0099] Embodiment 1, the acquisition of CYP76B74 protein and its coding gene
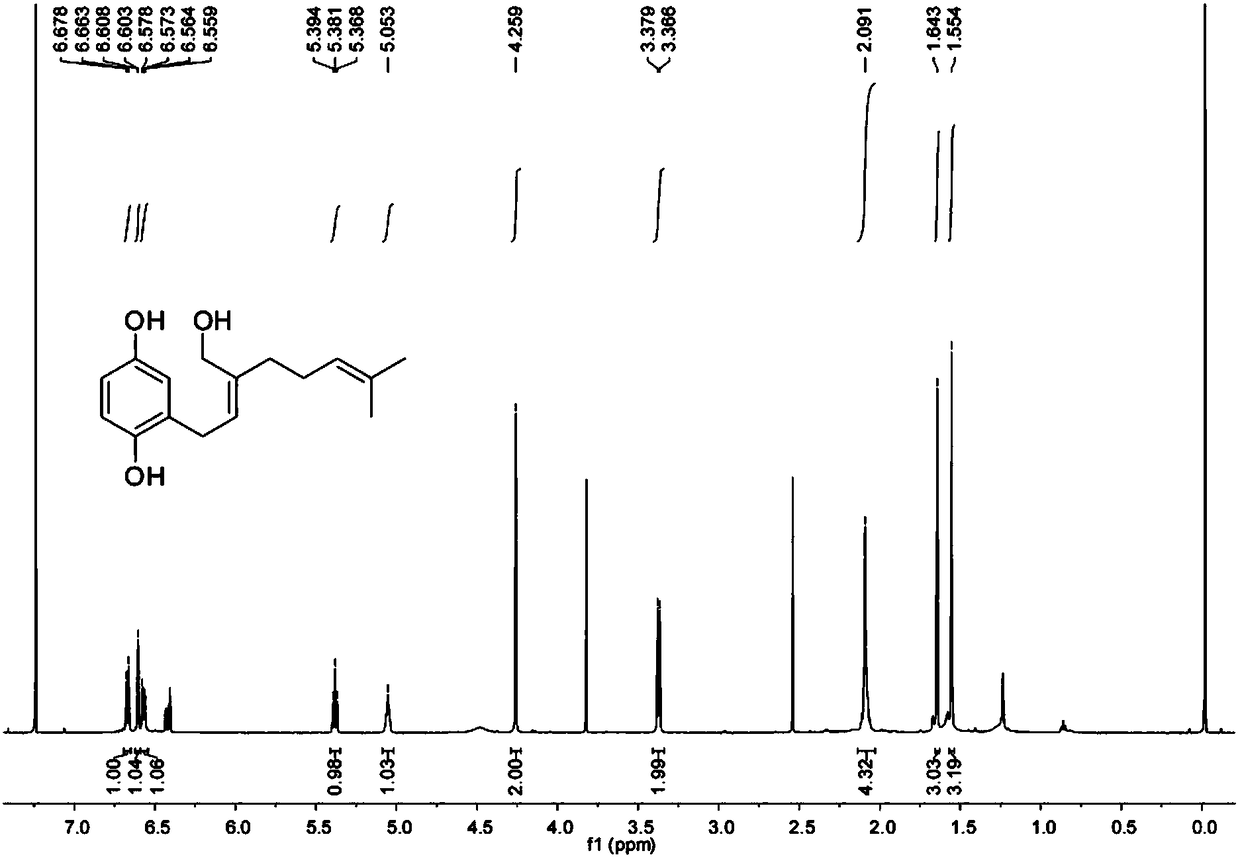
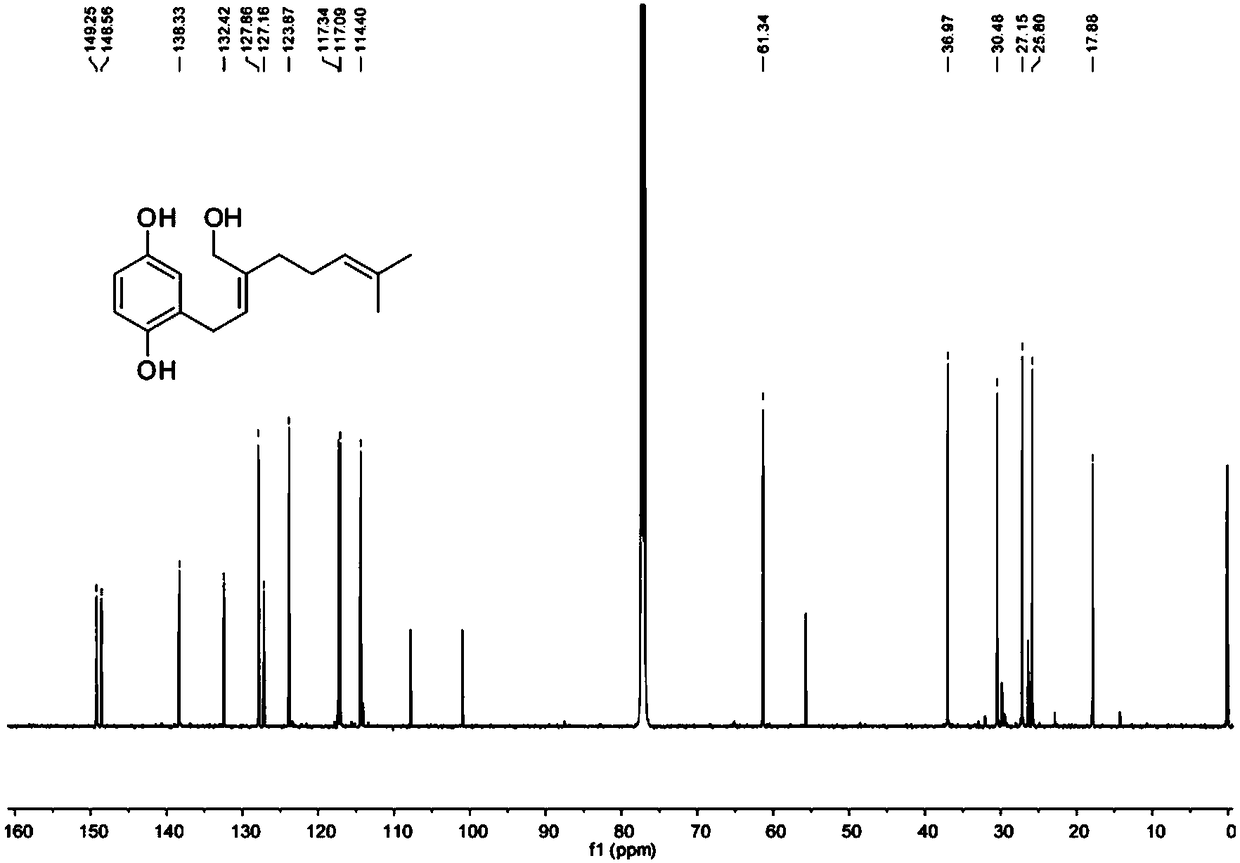
[0100] Through a large number of sequence analysis, expression analysis and functional verification of Zinnia xinjiang, a DNA coding sequence was found, and the encoded protein is shown in sequence 1 of the sequence list.
[0101] The protein shown in Sequence 1 of the Sequence Listing is named as CYP76B74 protein, which consists of 496 amino acid residues. The gene encoding CYP76B74 protein is named as CYP76B74 gene, and its open reading frame is shown as sequence 2 in the sequence listing.
Embodiment 2
[0102] Example 2, CYP76B74 protein function analysis
[0103] 1. Construction of recombinant strains
[0104] 1. Construction of recombinant plasmid pESC-Trp-CYP76B74. According to the sequencing results, the structure of the recombinant plasmid pESC-Trp-CYP76B74 is described as follows: the plasmid pESC-Trp is used as the starting vector, and the DNA molecule shown in sequence 2 of the sequence table is inserted before the FLAG tag.
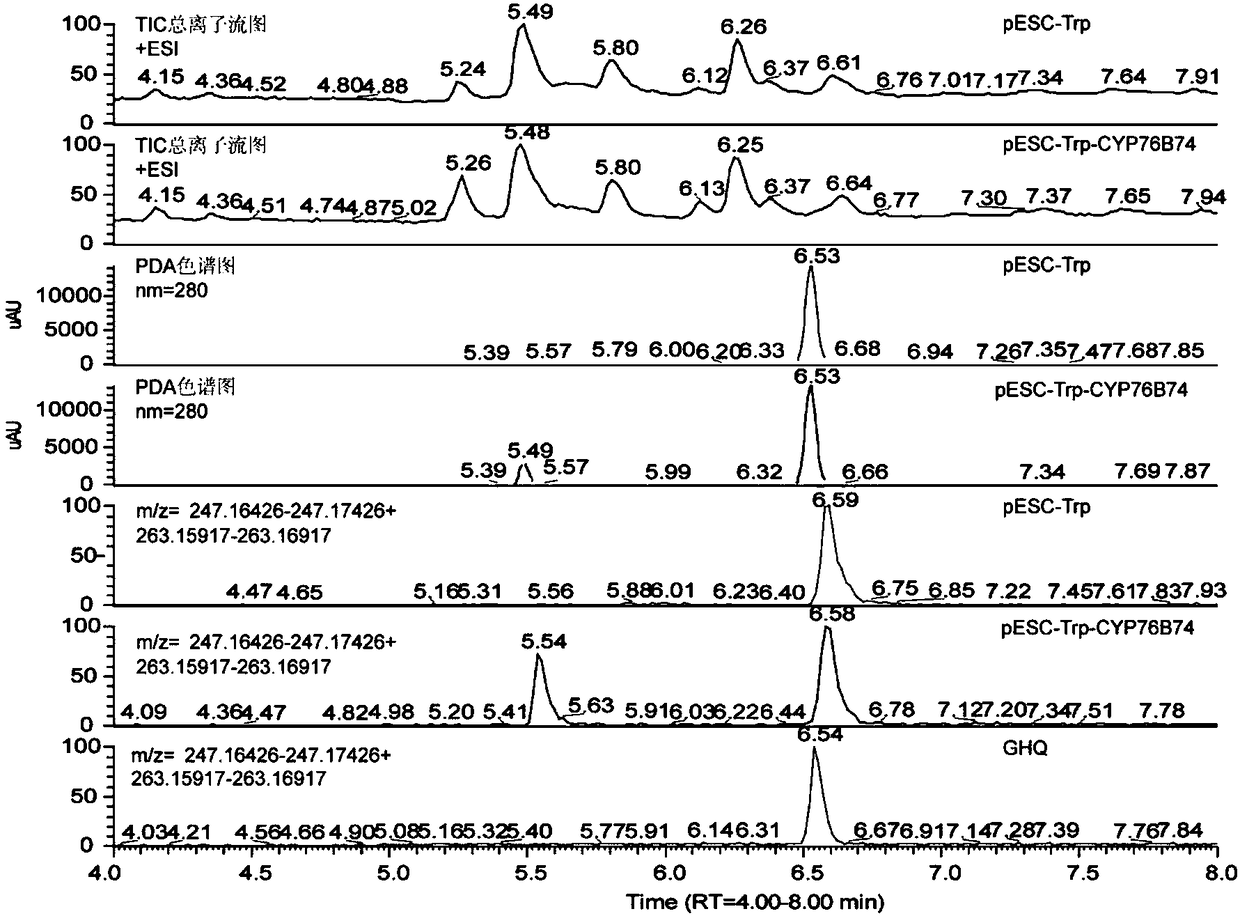
[0105] 2. Transform the recombinant plasmid pESC-Trp-CYP76B74 prepared in step 1 into Saccharomyces cerevisiae BY-T20 to obtain the recombinant strain BY-T20-CYP76B74; transform the plasmid pESC-Trp into Saccharomyces cerevisiae BY-T20 to obtain the recombinant strain BY-T20-Trp (hereinafter referred to as the empty vector strain).
[0106] 2. In vitro enzymatic experiment
[0107] TEG buffer: TE buffer containing 20% (v / v) glycerol.
[0108] Precipitation buffer: TESB buffer containing 0.225M NaCl and 0.15g / mL PEG4000.
[0109] TESB buff...
Embodiment 3
[0169] Example 3, Analysis of Enzymatic Kinetic Parameters of CYP76B74 Protein
[0170] 1. Prepare the reaction system. The reaction system is 1 mL, consisting of microsomal protein solution (500 μg protein content), Tris-HCl (pH7.5) 50 mM, NADPH 1 mM, FAD 5 μM, FMN 5 μM, 6-phosphate-glucose 50 mM, 6-phosphate-glucose dehydrogenation Enzyme 1U, GHQ and distilled water composition. The concentration of GHQ in the reaction system was 0 μM, 5 μM, 10 μM, 15 μM, 30 μM, 40 μM, 60 μM or 100 μM (each concentration was repeated three times).
[0171] 2. Take the reaction system prepared in step 1, and carry out an in vitro enzymatic reaction to obtain a reaction product.
[0172] Reaction conditions: 30°C, 150rpm for 30min.
[0173] 3. Add an equal volume of ethyl acetate to the reaction product, vortex for 1 min, centrifuge to collect the supernatant organic phase, and keep the lower bacterial liquid.
[0174] 4. Add an equal volume of ethyl acetate to the lower bacterial solution...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com