Production process of lambda-cyhalothric acid
A technology of trifluorochloro chrysanthemic acid and solvent, which is applied in the field of organic synthesis, can solve the problems of long cycle, low cis-trans ratio of cyclization reaction products, low production capacity, etc. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] (1) Addition: put 1250kg of methyl bentinate into the reaction kettle, 2500kg of trifluorotrichloroethane, 1250kg of solvent tert-butanol, 25kg of catalyst cuprous chloride, 60kg of 1-ethanolamine, heat up to about 100°C, 0.2 ~0.3MPa pressure-holding reaction for 12 hours, sampling analysis, control the content of pyridyl ester within 2%, steam trifluorotrichloroethane and tert-butanol under normal pressure after passing the test, and then steam the unreacted pyridyl ester under reduced pressure, The remaining adduct in the kettle is 2800kg, the content is more than 96%, and the yield is 95.6%.
[0032] (2) Cyclization: Add 1000kg of adduct, 2000kg of solvent N, N-dimethylformamide to the reaction kettle, control the temperature at -15°C, add 4000kg of sodium tert-amylate catalyst dropwise into the kettle, and finish adding in 30 minutes. Keep warm for 15 minutes after the dropwise addition, and take a sample for analysis. The content of the adduct is required to be 0.5...
Embodiment 2
[0036] (1) addition: the same as (1) addition step in Example 1.
[0037] (2) Cyclization: Add 1000kg of adduct to the reaction kettle, 2000kg of solvent N,N-dimethylacetamide, control the temperature at -15°C, add 4000kg of potassium tert-amylate catalyst dropwise into the kettle, and finish adding dropwise in 30 minutes. Keep warm for 15 minutes after the dropwise addition, and take a sample for analysis. The content of the adduct is required to be 0.5-1.0%. After passing the test, evaporate the solvent to dryness under negative pressure, add water to wash the cyclized product, and separate the lower layer of the cyclized product. The effective content is 94%, and the cis-trans ratio is 7.1:1. , yield 81.5%.
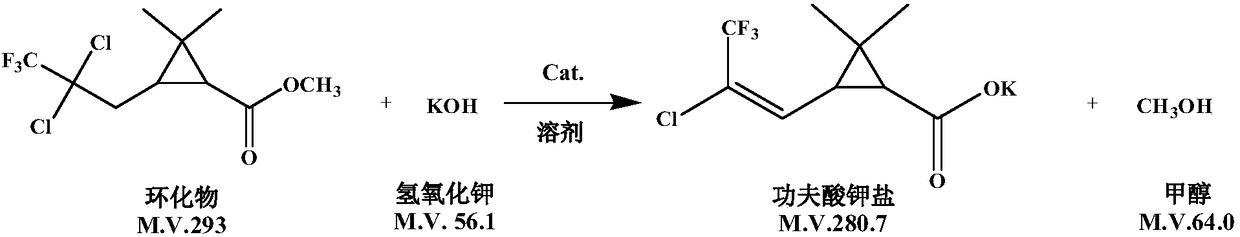
[0038] (3) Saponification: Add 5000kg of cyclized product, 9000kg of ethylene glycol, and 20kg of phase transfer catalyst tetrabutylammonium bromide into the reactor, stir and heat up to 90°C, and start to add 48% (w / w) KOH aqueous solution dropwise for 3 hours. After...
Embodiment 3
[0041] (1) addition: the same as (1) addition step in Example 1.
[0042] (2) Cyclization: Add 1000kg of adduct, 2000kg of solvent N,N-dimethylformamide to the reactor, control the temperature at -15°C, add 4000kg of sodium isoamyloxide catalyst dropwise into the kettle, and finish adding dropwise in 30 minutes. Keep warm for 15 minutes after the dropwise addition, and take a sample for analysis. The content of the adduct is required to be 0.5-1.0%. After passing the test, evaporate the solvent to dryness under negative pressure, add water to wash the cyclized product, and separate the lower cyclized product. The effective content is 92%, and the cis-trans ratio is 6.0:1 , yield 79.7%
[0043] (3) Saponification: Add 5000kg of cyclized product, 9000kg of n-butanol, 20kg of phase transfer catalyst tetrabutylammonium chloride into the reactor, stir and heat up to 90°C, start to add 48% (w / w) KOH aqueous solution dropwise for 3 hours After the addition, keep the temperature at 9...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com