Method for controlling sustained release of cyclodextrin inclusion compound in two-enzyme method
A technology of cyclodextrin inclusion compound and cyclodextrin, which is applied in the fields of food, medicine and textile and clothing, and can solve the problem that the guest molecules cannot be stably and completely utilized.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
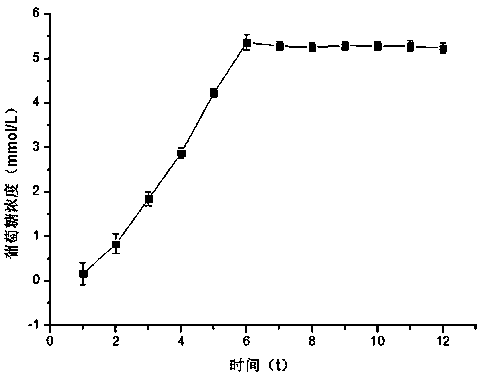
[0023] Example 1 The sodium benzoate-β-cyclodextrin inclusion complex is released uniformly, slowly and completely under the action of dual enzymes
[0024] (1) Dissolve 1.14 g (0.001 mol) of β-cyclodextrin in 100 mL of water, add 0.15 g (0.001 mol) of sodium benzoate to the aqueous solution of β-cyclodextrin, and stir at room temperature for 12 hours;
[0025] (2) Freeze-dry the solution obtained in step (1) at -50°C and 10Pa vacuum for 48 hours to obtain a mixture of sodium benzoate-β-cyclodextrin inclusion compound, and wash the powder with -4°C ultrapure water 3 times, until there is no sodium benzoate in the cold water;
[0026] (3) drying the solution in step (2) at 45°C to obtain sodium benzoate-β-cyclodextrin inclusion compound;
[0027] (4) dissolving the sodium benzoate-β-cyclodextrin inclusion compound in step (3) in a phosphate buffer solution with a pH of 5.5 to prepare a 1% reaction substrate solution;
[0028] (5) Take 900 μL of the solution from step (4) in a...
Embodiment 2
[0029] Example 2: Uniform, slow and complete release of vanillin-β-cyclodextrin inclusion compound under the action of dual enzymes
[0030] (1) Dissolve 1.14 g of β-cyclodextrin in 100 mL of water, add 0.15 g of vanillin to the aqueous solution of β-cyclodextrin, and stir for 12 hours at room temperature;
[0031] (2) Freeze-dry the solution obtained in step (1) at -50°C and 30Pa vacuum for 24 hours to obtain a mixture of vanillin-β-cyclodextrin inclusion compound, wash the powder 3 times with ethanol, and put it in ethanol No vanillin present;
[0032] (3) drying the step (2) at 25°C to obtain the inclusion compound of vanillin-β-cyclodextrin;
[0033] (4) Dissolve the powder of step (3) in a phosphate buffer solution with a pH of 5.5 to make a 1% reaction substrate solution;
[0034] (5) Take 900 μL of the solution from step (4) in a centrifuge tube, add 200 μL CGTase and 200 μL glucoamylase for reaction, and the guest molecule vanillin will be released uniformly and comp...
Embodiment 3
[0035] Example 3: Uniform, slow and complete release of β-carotene-γ-cyclodextrin inclusion compound under the action of dual enzymes
[0036] (1) Dissolve 2.27 g of γ-cyclodextrin in 100 mL of water, add 0.53 g of β-carotene n-hexane solution to the aqueous solution of γ-cyclodextrin, and stir for 12 hours at room temperature in the dark;
[0037] (2) Freeze-dry the liquid obtained in step (1) at -50°C and 20Pa vacuum for 36 hours to obtain a mixture of β-carotene-γ-cyclodextrin inclusion compound, wash the powder 3 times with ethanol, and put it in ethanol No β-carotene present;
[0038] (3) drying the solution in step (2) in the dark at 30°C to prepare β-carotene-γ-cyclodextrin inclusion compound;
[0039] (4) Dissolve the powder of step (3) in a phosphate buffer solution with a pH of 5.5 to make a 1% reaction substrate solution;
[0040] (5) Take 900 μL of the solution from step (4) in a centrifuge tube, add 10 μL CGTase and 10 μL glucoamylase to react, and the guest mol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com