Haemadipsa sylvestris antithrombosis polypeptide Sylvestin as well as in-vitro expression preparation method and application thereof
An anti-thrombotic and leech technology, applied in botany equipment and methods, biochemical equipment and methods, applications, etc., can solve the problems of few types of polypeptides, low activity, instability, etc., and achieve uniform conformation, high stability, The effect of high enterokinase cleavage efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0045] The present invention also provides a preparation method for the in vitro expression of the polypeptide Sylvestin, comprising the following steps: 1) constructing the recombinant expression strain according to claim 6; 2) inducing the expression of the recombinant strain to obtain a bacterial solution containing the recombinant polypeptide Sylvestin; The induction temperature is 16-30°C, the induced IPTG concentration is 0.05-0.55mmol / L, and the induction time is 1-18h; 3) Separating and purifying the recombinant polypeptide Sylvestin from the bacterial liquid; 4 ) cutting the recombinant polypeptide Sylvestin with enterokinase to obtain the polypeptide Sylvestin.
[0046] In the present invention, the method for constructing the recombinant expression strain comprises the following steps: a, linking the enterokinase cleavage site with the nucleotide sequence encoding the polypeptide Sylvestin to obtain the nucleotide sequence encoding the recombinant polypeptide Sylvest...
Embodiment 1
[0066] Obtaining the Antithrombotic Polypeptide Sylvestin from Natural Forest Leech
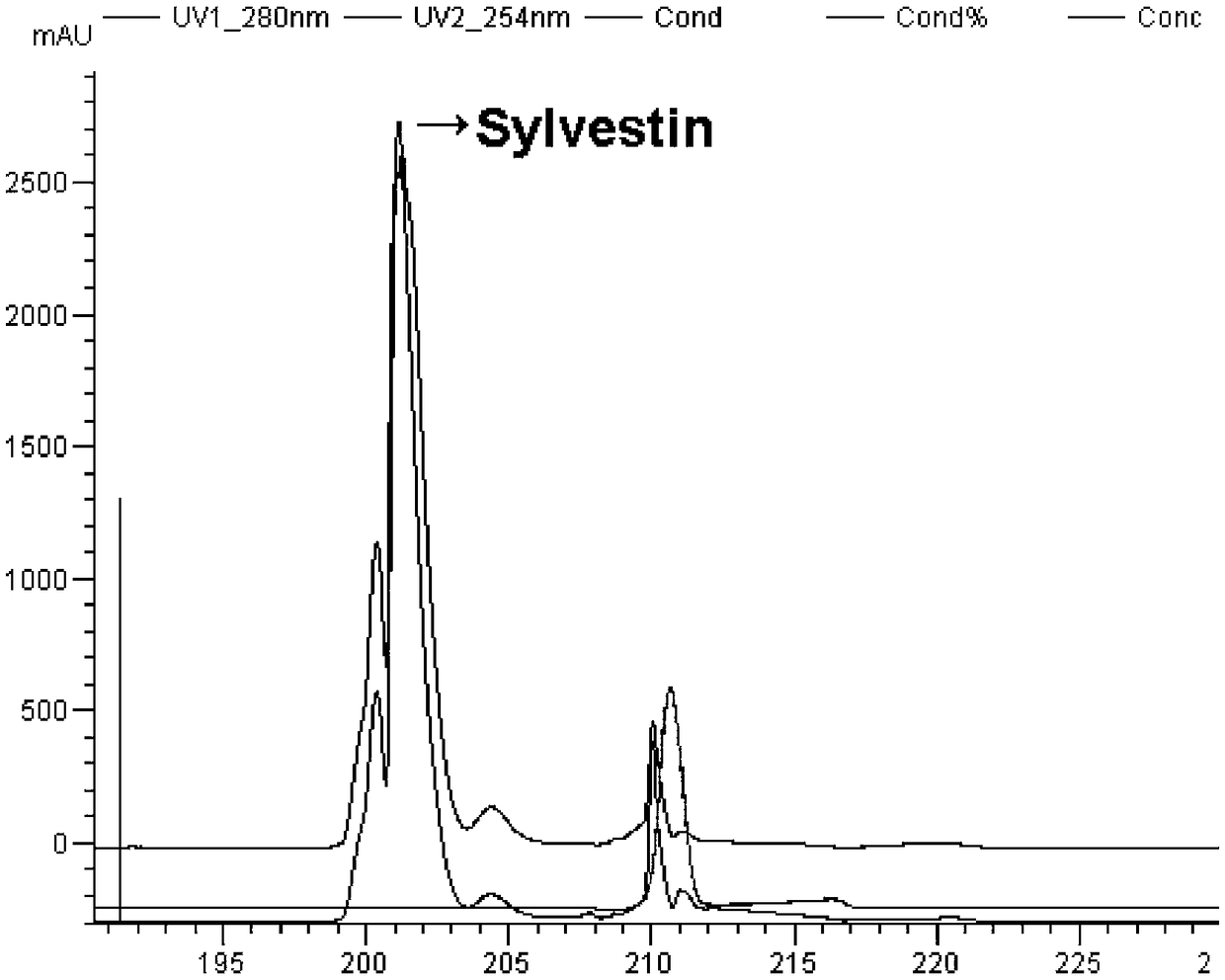
[0067] Polypeptide Sylvestin was isolated and purified from the whole homogenate of Leech leech by molecular sieve gel filtration and reversed-phase high-pressure liquid chromatography. The N-terminal 12 amino acid sequences of the polypeptide Sylvestin were determined by the Edman degradation method, and the homologous sequence encoding the polypeptide was screened from the Yunnan forest leech transcriptome, and the homologous nucleotide sequence was used as a template to design a specific Sex primers, the forward primer is AAACCGAACCGTCGGTATGT, the reverse primer sequence is
[0068] GTTCGGTTTGGTGGCTCATT. Then the nucleotide sequence encoding polypeptide Sylvestin was amplified from the constructed leech head cDNA library.
[0069] The mature peptide encoded by the nucleotide sequence is composed of 43 amino acids, contains six cysteines, and has a molecular weight of 4795.4 Da, which is ...
Embodiment 2
[0071] A wood leech antithrombotic recombinant polypeptide Sylvestin, including the polypeptide Sylvestin and an enterokinase cleavage site fragment, the sequence is shown in Seq ID No. 3, specifically MKLLVVLLIVSLVGMSHQTSEPVCAPKMLFWVCGKDGETYTHPCIAKCHNVEVEHDGKCK. The recombinant polypeptide Sylvestin described in the present invention is connected with an enterokinase enzyme cleavage site on the sequence of the polypeptide Sylvestin, which facilitates the connection of the polypeptide Sylvestin to the carrier and the separation and purification of the polypeptide Sylvestin during the preparation process of in vitro expression.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Relative molecular mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com