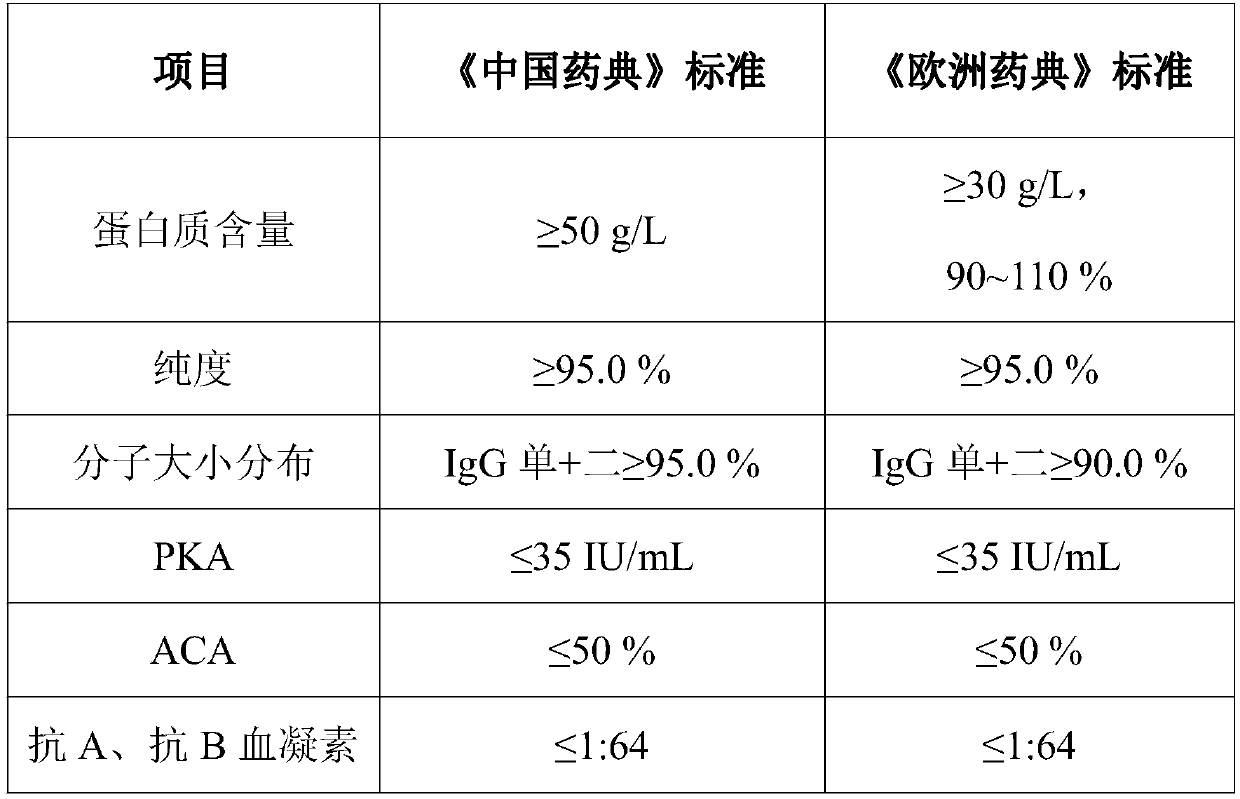
A kind of production technology of intravenous immunoglobulin
An immunoglobulin and production process technology, applied in the field of biopharmaceuticals, can solve the problems of easy to exceed, high ACA content, easy to appear natural crystallization, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
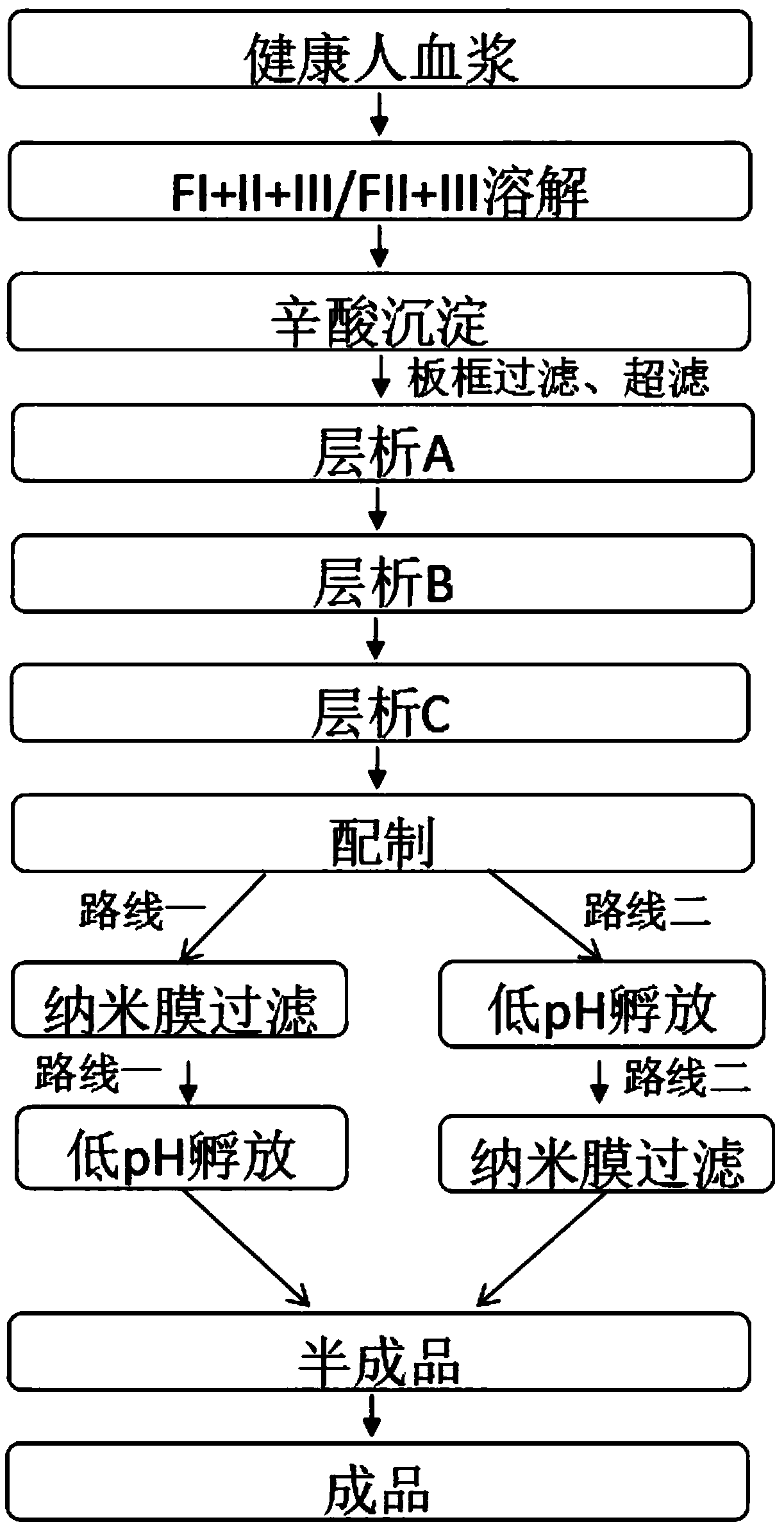
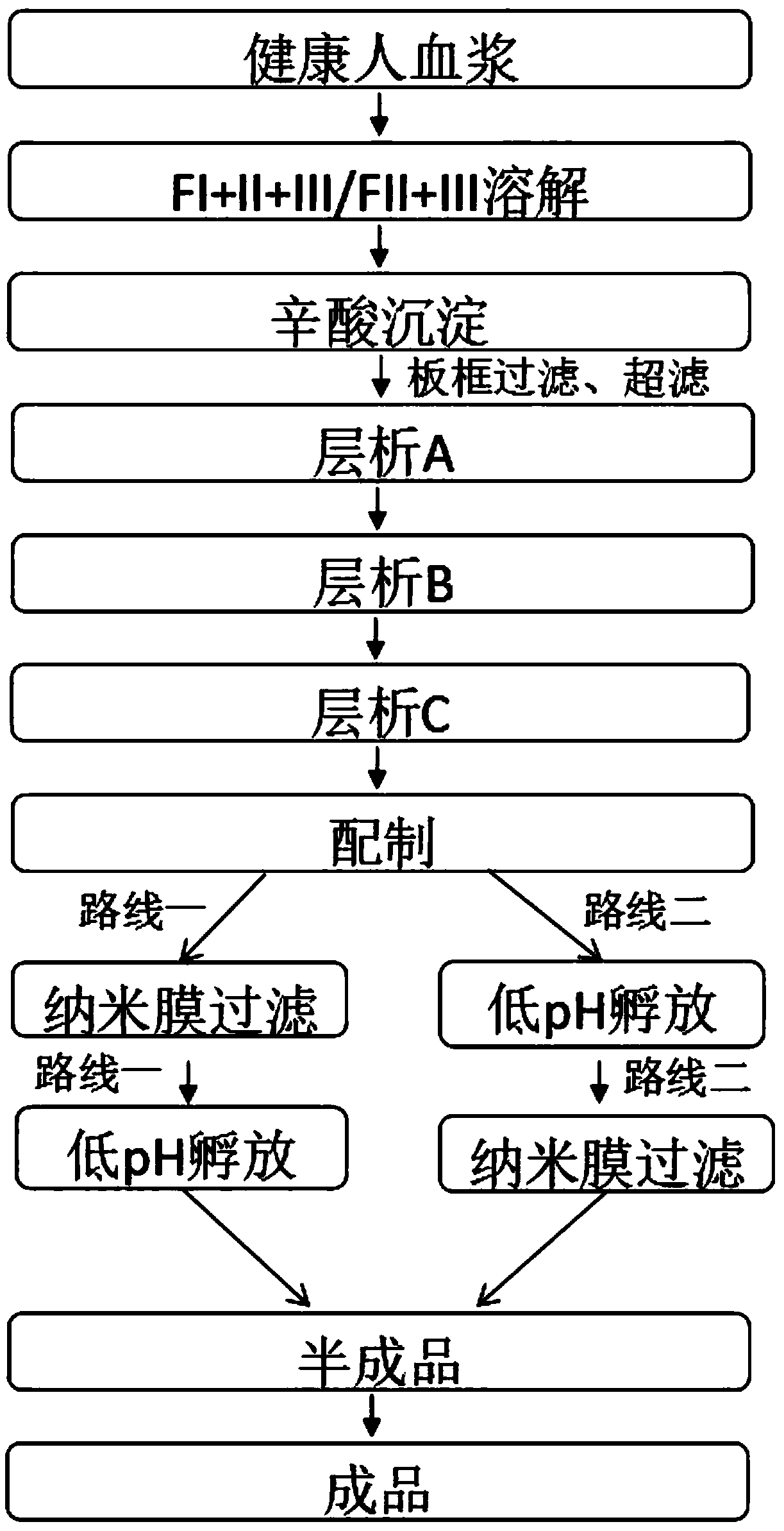
[0042] A kind of production technology of intravenous immunoglobulin, is according to such as figure 1 The production process of the middle route one is carried out, which specifically includes the following steps:
[0043] (1) The plasma of healthy people was prepared by low-temperature ethanol reaction to obtain the precipitate of component I+II+III, and then the precipitate of component I+II+III was dissolved step by step with 10 times of WFI, and the temperature of the solution was controlled at 2°C. Adjust the pH to 4.00 and stir for 1h;
[0044] (2) Caprylic acid precipitation: adjust the pH of the solution to 4.80 with NaOH, add caprylic acid dropwise to the solution within 40 minutes to a final concentration of 10 mM, after the addition of caprylic acid, adjust the pH to 4.80 with NaOH, stir for 1 hour, and filter the plate and frame. Obtain the clarified liquid after octanoic acid precipitation;
[0045] (3) Chromatography A: choose XK50 / 30 chromatographic column to...
Embodiment 2
[0051] according to figure 1 The production process of the middle route two prepares intravenous immunoglobulin products, and its operation steps are as follows:
[0052] (1) Precipitation of component II+III was prepared by reacting healthy human plasma with ethanol at low temperature, and then the precipitation of component II+III was dissolved step by step with 25 times of WFI. The temperature of the solution was controlled at 25°C, and the pH was adjusted to 5.00 with hydrochloric acid. , stirred for 3h;
[0053] (2) Octanoic acid precipitation: adjust the pH of the solution to 5.80 with NaOH, add octanoic acid dropwise to the solution within 60 minutes to a final concentration of 30 mM, after the addition of octanoic acid is completed, adjust the pH to 5.80 with NaOH, stir for 3 hours, and then plate and frame filter, Obtain the clarified liquid after octanoic acid precipitation;
[0054] (3) Chromatography A: Choose XK50 / 30 chromatographic column to pack 23cm high Frac...
Embodiment 3
[0060] according to figure 1 The production process of route 1 and the specific operation steps in Example 1 are prepared from the precipitation of components I+II+III as raw materials to prepare IgG concentrate products. The differences in operation are as follows:
[0061] In step (1), 18 times of WFI is used to gradually dissolve the precipitate of component I+II+III, control the temperature of the solution to 15°C, adjust the pH to 4.35, and stir for 2 hours;
[0062] In step (2), adjust the pH of the solution to 5.20, add caprylic acid dropwise to the solution to a final concentration of 22 mM, stir for 2 hours and then filter;
[0063] In step (3), the loading capacity of Fractogel EMD DEAE gel is 350g precipitation / 400mL gel, and the column height is 20cm. The clarified liquid is filtered and concentrated until the protein content is 5g / L. The conditions of Fractogel EMD DEAE chromatography are: pH 4.70, 8mM sodium acetate buffer solution, conductance 0.8ms / cm, balance...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com