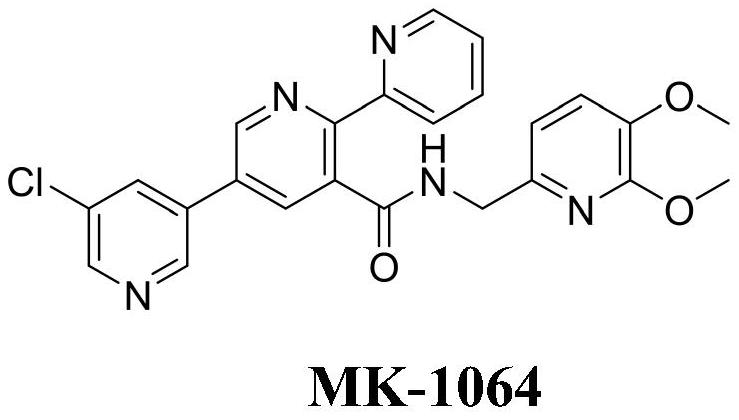
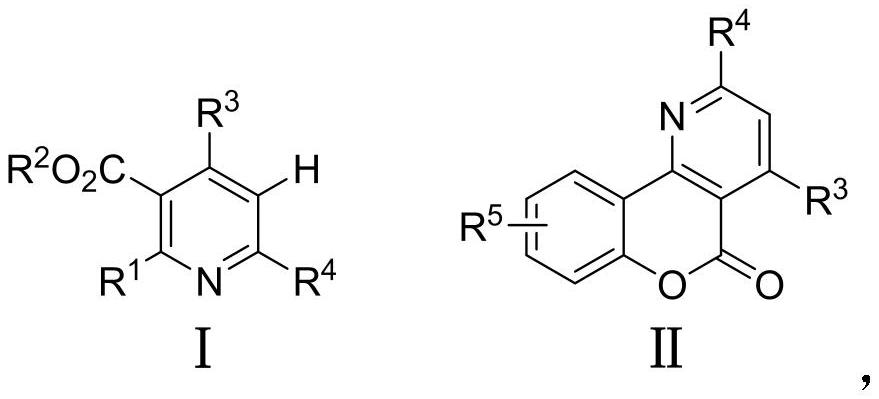
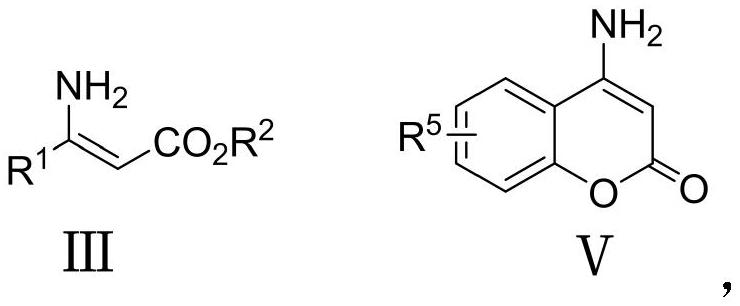
A kind of preparation method and application of multi-substituted pyridine derivatives
A derivative and multi-substitution technology, which is applied in the field of preparation of multi-substituted pyridine derivatives, can solve the problems of harsh reaction conditions, complicated experimental operations, and difficult availability of starting materials, and achieve high reactivity, simple experimental operations, and excellent reaction conditions. mild effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Embodiment 1: Preparation of 6-(4-bromophenyl)-2-phenylnicotinic acid ethyl ester (Ia)
[0033] Ethyl 3-amino-3-phenylacrylate (0.96g, 5mmol), p-bromopropiophenone (1.28g, 6mmol), copper acetate (0.18g, 1mmol), bpy (0.31g, 2mmol) and 4-OH -TEMPO (0.86g, 5mmol) in toluene (10mL) was stirred at 120°C for 24 hours. After the reaction, it was extracted with water and ethyl acetate. The organic phase was dried over anhydrous sodium sulfate, concentrated, and washed with petroleum ether / acetic acid Ethyl ester = 50 / 1 (V / V) mixed solvent as eluent, separated by silica gel column chromatography to obtain 1.59g of white solid 6-(4-bromophenyl)-2-phenylnicotinic acid ethyl ester (Ia) , yield: 83%, melting point: 59-60°C.
[0034] The structural formula of Ia is:
[0035]
[0036] 1 H NMR (600MHz, CDCl 3 ,ppm)δ8.18(d,J=7.8Hz,1H),8.01(d,J=8.4Hz,2H),7.75(d,J=7.8Hz,1H),7.63-7.60(m,4H), 7.46(m,3H),4.17(q,J=7.2Hz,2H),1.07(t,J=7.2Hz,3H). 13 C NMR (150MHz, CDCl 3 ,ppm)δ168.2,15...
Embodiment 2
[0037] Example 2: Preparation of ethyl 6-(4-bromophenyl)-2-(2-thienyl)nicotinate (Ib)
[0038] 3-Amino-3-(2-thienyl)enoic acid ethyl ester (0.91g, 5mmol), p-bromopropiophenone (1.07g, 5mmol), copper acetate (0.18g, 1mmol), bpy (0.16g, 1mmol) ) and 4-OH-TEMPO (1.29g, 7.5mmol) in toluene (10mL) were stirred at 110°C for 30 hours. After the reaction, extracted with water and ethyl acetate, the organic phase was dried over anhydrous sodium sulfate and concentrated , using petroleum ether / ethyl acetate=50 / 1 (V / V) mixed solvent as eluent, separated by silica gel column chromatography to obtain white solid 6-(4-bromophenyl)-2-(2-thienyl ) ethyl nicotinate (Ib) 1.30 g, yield: 70%. Melting point: 65.5-66.2°C.
[0039] Ib structural formula is:
[0040]
[0041] 1 H NMR (600MHz, CDCl 3 ,ppm)δ8.03-8.01(m,3H),7.66-7.63(m,3H),7.50-7.48(m,2H),7.13-7.11(m,1H),4.40(q,J=7.2Hz, 2H), 1.34(t, J=7.2Hz, 3H). 13 C NMR (150MHz, CDCl 3 , ppm) δ168.3, 156.7, 150.5, 143.2, 138.7, 136.7, 132.0, ...
Embodiment 3
[0042] Example 3: Preparation of tert-butyl 6-(4-bromophenyl)-2-benzyl nicotinate (Ic)
[0043] tert-butyl 3-amino-3-phenylacrylate (1.10g, 5mmol), p-bromopropiophenone (3.20g, 15mmol), copper acetate (0.18g, 1mmol), 1,10-Phen (0.20g, 1mmol ) and TEMPO (5mmol, 0.78g) in toluene (10mL) were stirred and reacted at 130°C for 20 hours. After the reaction, extracted with water and ethyl acetate, the organic phase was dried with anhydrous sodium sulfate, concentrated, and washed with petroleum ether / Ethyl acetate=50 / 1 (V / V) mixed solvent was used as eluent, separated by silica gel column chromatography to obtain white solid 6-(4-bromophenyl)-2-benzyl nicotinic acid tert-butyl ester (Ic ) 1.01g, yield: 49%.
[0044] The structural formula of Ic is:
[0045]
[0046] 1 H NMR (600MHz, CDCl 3 ,ppm)δ8.19(d,J=7.8Hz,1H),8.02(d,J=9.0Hz,2H),7.75(d,J=8.4Hz,1H),7.53(dd,J=7.8,1.8 Hz,2H)7.63(d,J=9.0Hz,2H),7.51-7.46(m,3H),1.35(s,9H). 13 C NMR (150MHz, CDCl 3 , ppm) δ167.3, 158.7, 156.8...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com