Cyanine-based organic compound, intermediate and application thereof
An organic compound and compound technology, applied in the field of fluorescent probes, can solve the problems of in situ and real-time detection of organisms, and achieve the effects of short response time, reduced interference and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Preparation of Cyanine-Based Organic Compounds:
specific Embodiment
[0034] The present invention is a cyanine-based organic compound. The cyanine fluorophore shown in the compound of formula I is commercially available, and then different detection groups are modified at the designated positions of the fluorophore to obtain the corresponding cyanine compound. Specific examples are as follows:
[0035] (1) Preparation of compound II
[0036] Dissolve p-methylaminonitrobenzene (0.06 g, 0.4 mmol) in a 20 ml anhydrous DMF round-bottomed flask, add 0.0167 g of sodium hydride at room temperature, and stir for 15 minutes under the protection of argon. Then, cyanine fluorophore (0.255 g, 0.4 mmol) was added to the above solution and reacted at room temperature for 1 hour. Wash with excess saturated potassium iodide solution, extract with dichloromethane after washing, and rotary evaporate to obtain crude product. The crude product was purified by column chromatography, and the eluent was selected as dichloromethane:methanol=1:4 to obtain compound II...
Embodiment 2
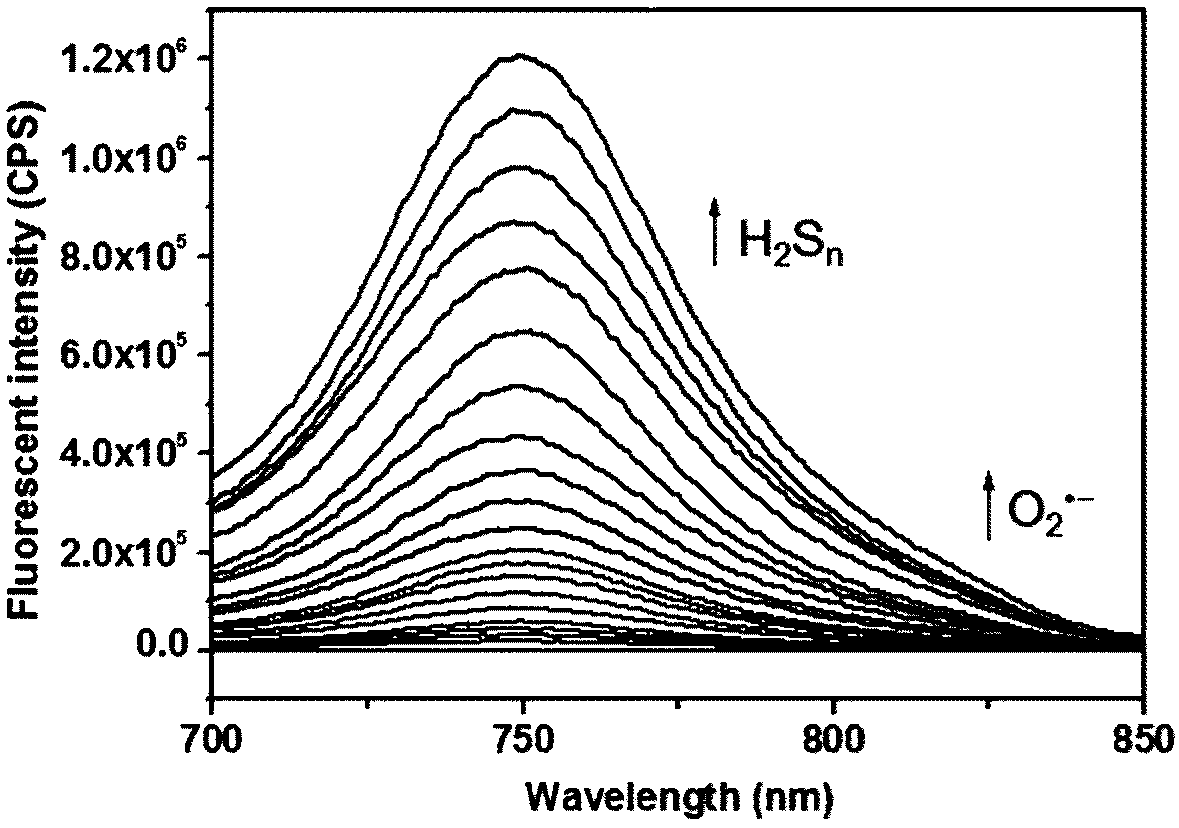
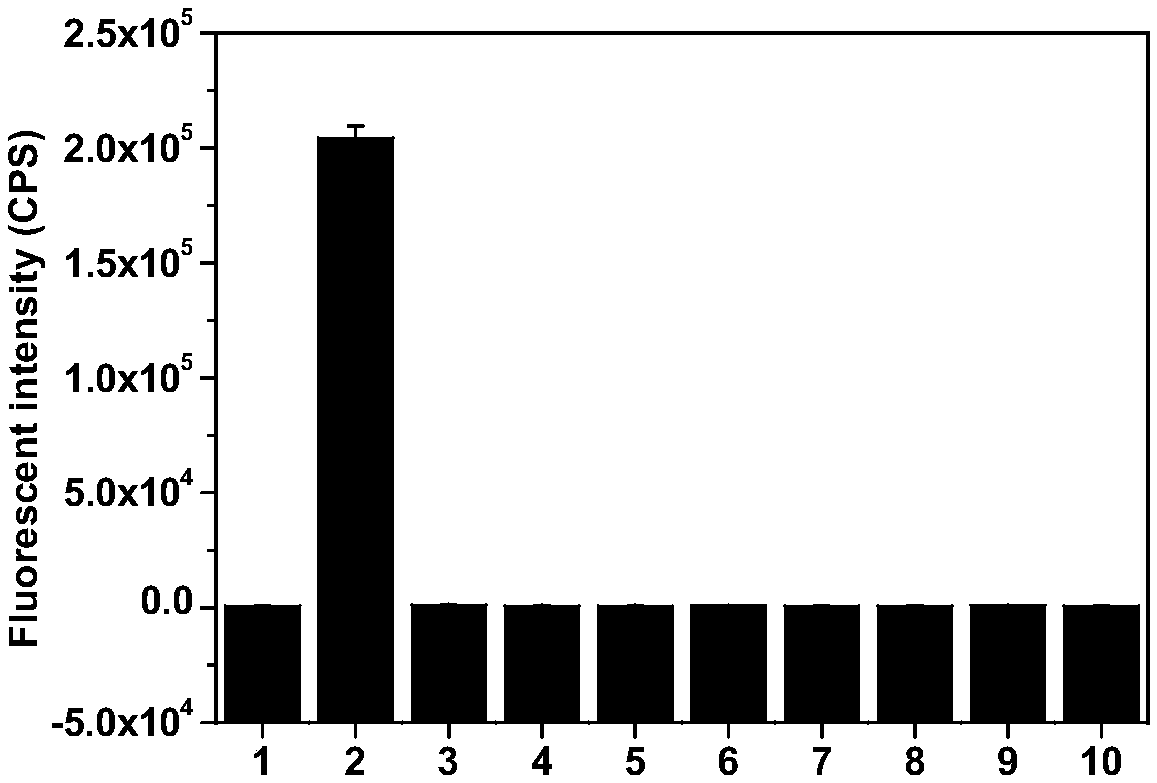
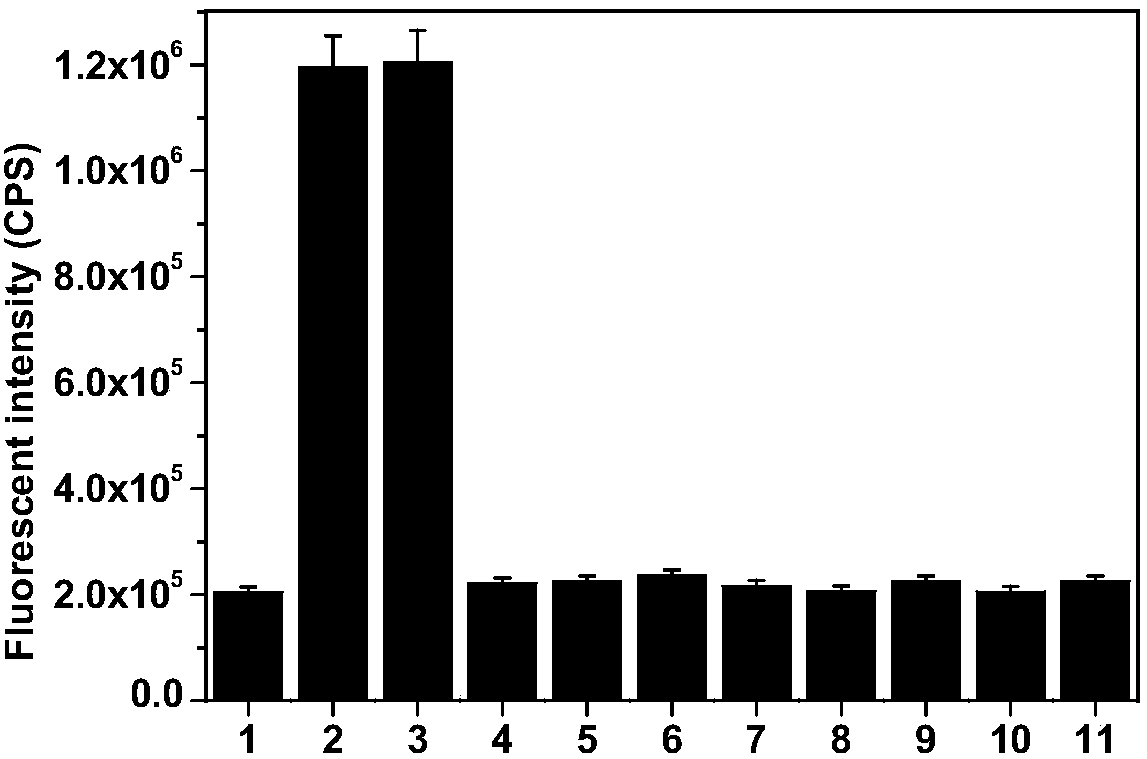
[0042] The prepared compound of formula I was used as a fluorescent probe in water system, simulated physiological environment and living cells for O 2 ·- and H 2 S n The detection was carried out to simulate physiological conditions. The following experiments were all carried out under the condition of pH=7.4 (HEPES buffer solution, the concentration was 10 mM), and the probe concentration was 10 μM. 1 μM probe was used in cell experiments.
[0043] Above-mentioned preparation gained formula I compound is as probe pair O 2 ·- the response to:
[0044] Add different concentrations of O to each 10ml colorimetric tube 2 ·- (0-10 μM), the volume was adjusted to 10 ml with 10 mM HEPES (pH 7.4), and finally the compound of formula I was added (final concentration 10 μM). Shake the solution well, and after equilibrating at 25°C for 15 minutes, pipette 1ml of the solution into a fluorescent dish to measure the fluorescence spectrum. Fluorescence spectra were measured at 700-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com