Method for selectively producing ginsenoside rd from saponins of ginseng through enzymatic method
A ginsenoside and ginseng technology are applied in the field of selectively preparing ginsenoside Rd from ginsenoside by an enzymatic method, which can solve the problems of ginsenoside only increasing the price, restricting the large-scale use of ginsenoside, consuming cost, time and the like.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
reference example 1
[0034] [Reference Example 1] Preparation of Refined Ginseng Saponins
[0035] Put 2kg of red ginseng, white ginseng, fresh ginseng, ginseng whiskers or their ginseng leaves, ginseng flowers, and ginseng fruits into 20L of ethanol, reflux extraction for 3 times, and settle at 15°C for 6 days. Afterwards, the residue and filtrate were separated by filter cloth filtration and centrifugation, the concentrated solution obtained by concentrating the separated filtrate under reduced pressure was suspended in water, extracted 5 times with 1L ether to remove the pigment, and the water layer was extracted with 1L of 1 - Butanol extraction 3 times. All the 1-butanol layers thus obtained were treated with 5% KOH, washed with distilled water, and then concentrated under reduced pressure to obtain a 1-butanol concentrate, which was dissolved in a small amount of methanol, and then a large amount of ethyl acetate was added Esters, by drying the precipitate generated, 40-80g of refined ginse...
Embodiment 1
[0036] [Example 1] Preparation of Ginsenoside Rd by Enzyme Reaction
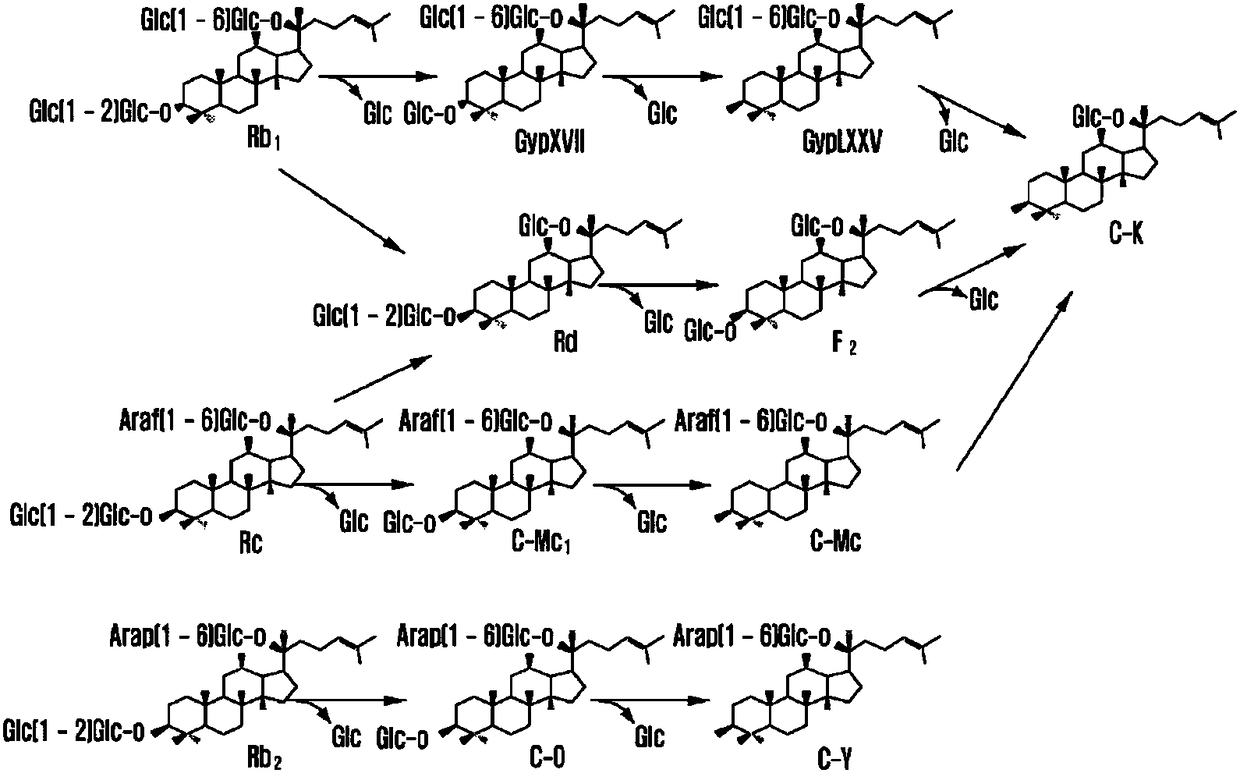
[0037] Dissolve 10 g of the refined ginsenosides (containing ginsenosides Rb1, Rb2, Rc, Rd, Re, Rg1, Rf, etc.) in Reference Example 1 above in 1 L of water.
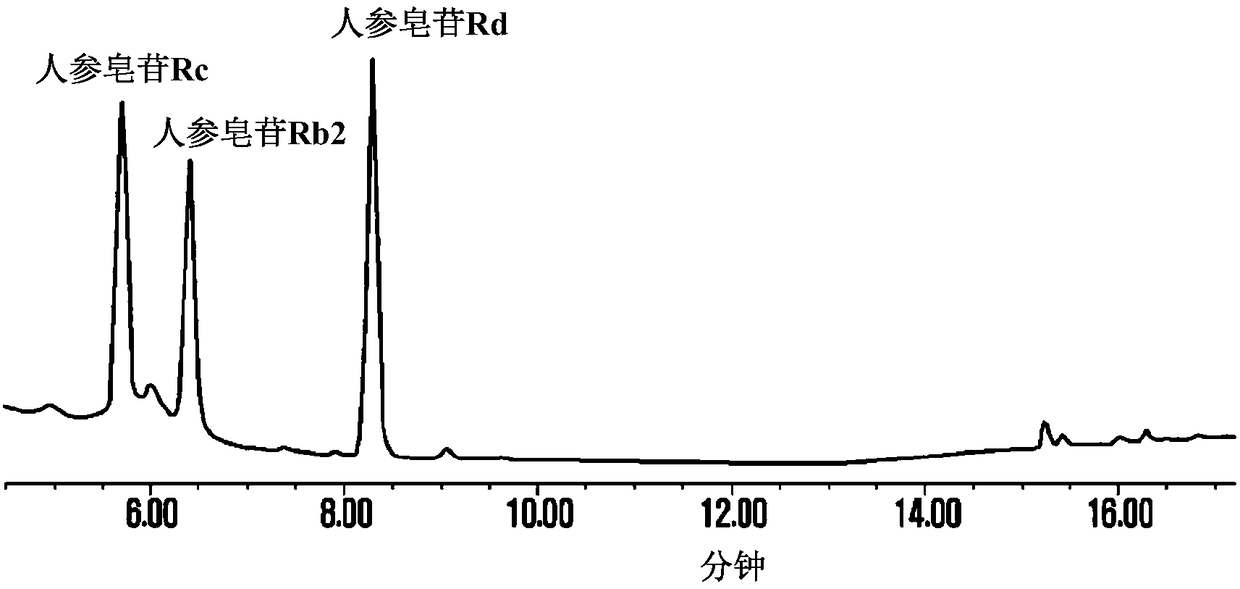
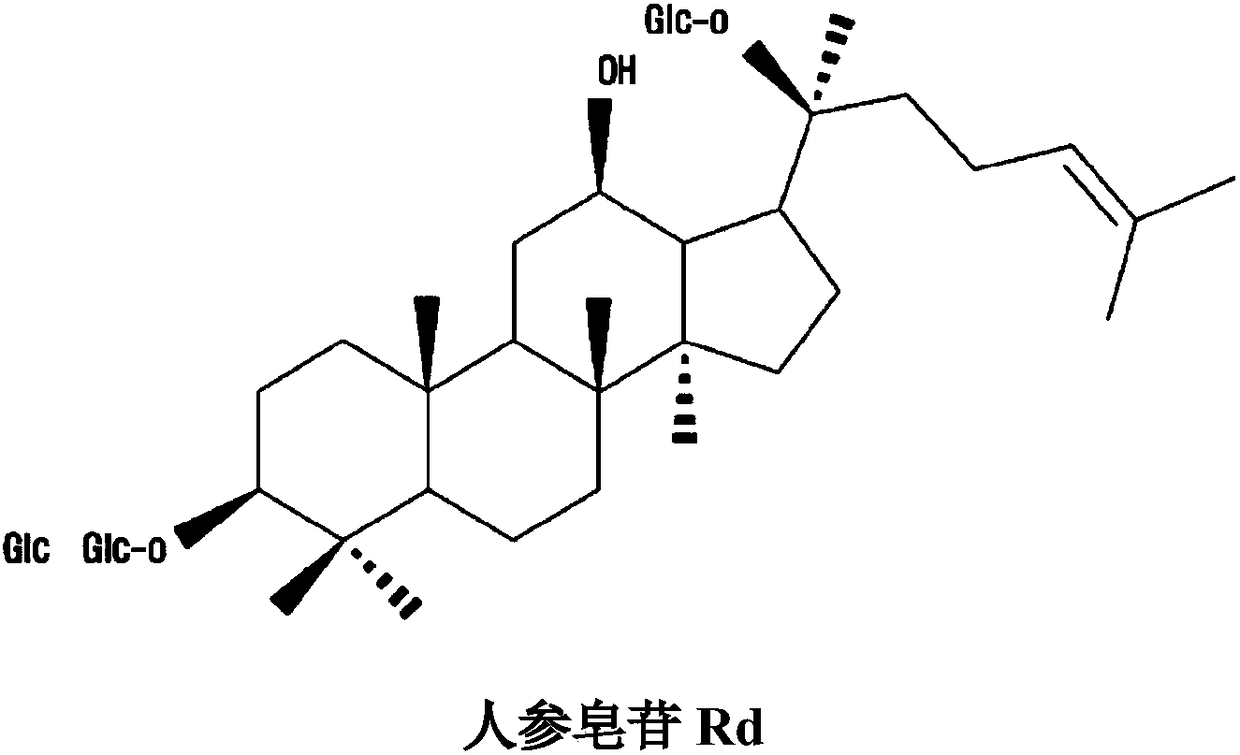
[0038] Then, 200% by weight of the pectinase isolated from Trichoderma reesei was added to the above-mentioned mixed liquid, and then reacted at 30° C. for 48 hours. Periodically confirm the complete consumption of the substrate by thin-layer chromatography, inactivate the enzyme by heating in a boiling water bath for 10 minutes, and terminate the reaction. Finally, add ethyl acetate or ethanol to the reaction solution at a ratio of 1:1 (relative to the volume ratio of the reaction solution), extract 3 times, concentrate, and separate by silica gel column chromatography (chloroform:methanol=9:1). Ginsenoside Rd( figure 1 ).
[0039] In 10 g of refined ginsenosides used there were: 2.66 g of ginsenoside Rb1, 0.73 g of ginsenoside Rb2, 1.23 g of ginse...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com