Preparation method of recombinant anti-TNF-alpha completely humanized monoclonal antibody
A monoclonal antibody, fully human technology, applied in the direction of recombinant DNA technology, anti-animal/human immunoglobulin, chemical instruments and methods, etc., can solve the problem of antibody expression, stability, titer difference, technical means level Unevenness, the quality of life of patients cannot be effectively guaranteed, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1 Obtainment and Synthesis of Light Chain and Heavy Chain Sequences
[0028] The present invention determines the IgG1 light chain constant region kappa sequence (IGK, Gene ID: 50802) and heavy chain constant region gamma sequence (IGHG1, Gene ID: 3500) of IgG1 according to human IgG1 related literature and GeneBank data, and according to the original drug Humira Publicly available data identified the amino acid and DNA sequences of the light and heavy chain variable regions of adalimumab. After sequence comparison and confirmation, the codons preferred by Chinese hamsters were selected to artificially synthesize the full-length light chain L, heavy chain variable region VH and heavy chain constant region CH sequences, and the required enzyme cutting sites and protection sites were added.
[0029] Wherein the nucleotide sequence of the light chain has the sequence of SEQ ID NO.1, the amino acid sequence of the light chain has the sequence of SEQ ID NO.3; the nuc...
Embodiment 2
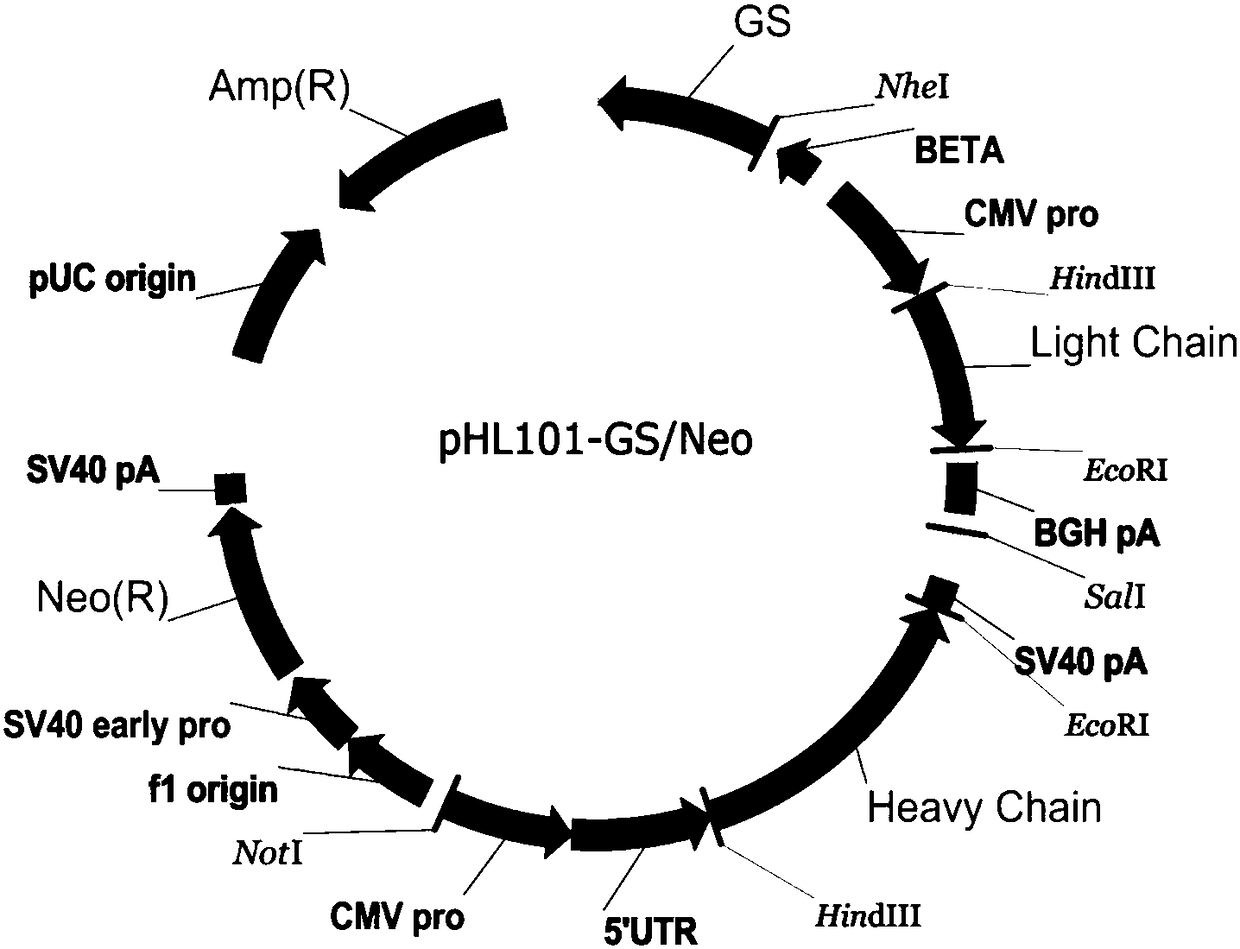
[0030] Embodiment 2pHL101-GS / Neo vector construction
[0031] (1) The synthetic light chain full-length sequence L, heavy chain variable region VH and heavy chain constant region CH sequences are placed in the amplification plasmid pEASY, and amplified in large quantities to obtain sufficient target fragments.
[0032] (2) First select heavy chain constant region CH double enzyme digestion (HindⅢ and NheI) and connect it into a mammalian expression vector such as pcDNA3.1(+) to obtain the connection vector of heavy chain constant region CH, which is convenient for integration with other heavy chain The V regions are connected. A double BbsI structure restriction site (gtcttcgagaagac) is added to the N-terminus of the CH sequence, which is connected to the heavy chain V region. The restriction endonuclease characteristics of BbsI are different from those of traditional restriction endonucleases. The base at the position forms a gap with a difference of 4 bases. In the case of...
Embodiment 3
[0035] Embodiment 3 transfection host cell CHO-GS -Screening for high expression clones
[0036] CHO cells can be grown in high density in bioreactors, easy to perform genetic manipulation, N-glycosylation type is similar to human beings, and have low risk of virus transmission, so they are widely used in the field of biopharmaceuticals. The present invention uses the GS-deficient cell CHO-GS independently transformed and identified by our commercialized CHO-K1 cell line - , to further reduce the expression of endogenous GS, using the GS-Neo dual screening system.
[0037] The linearized pHL101-GS / Neo plasmid was transfected by electroporation, and the GS-Neo dual screening system was used for gradient pressurization screening to obtain stable cell clones that highly express recombinant anti-TNF-α fully human monoclonal antibody. After multiple rounds of transfection and screening, after identification and detection, cell clones with expression levels greater than 35pg / cell·...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com