Method for stabilizing iron arsenate slag
A technology of iron arsenate slag and iron arsenate, which is applied in the field of arsenic pollution control, can solve the problems of unmentioned arsenic-containing sludge treatment and complicated preparation process, and achieve the effects of low cost, simple preparation and wide sources
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
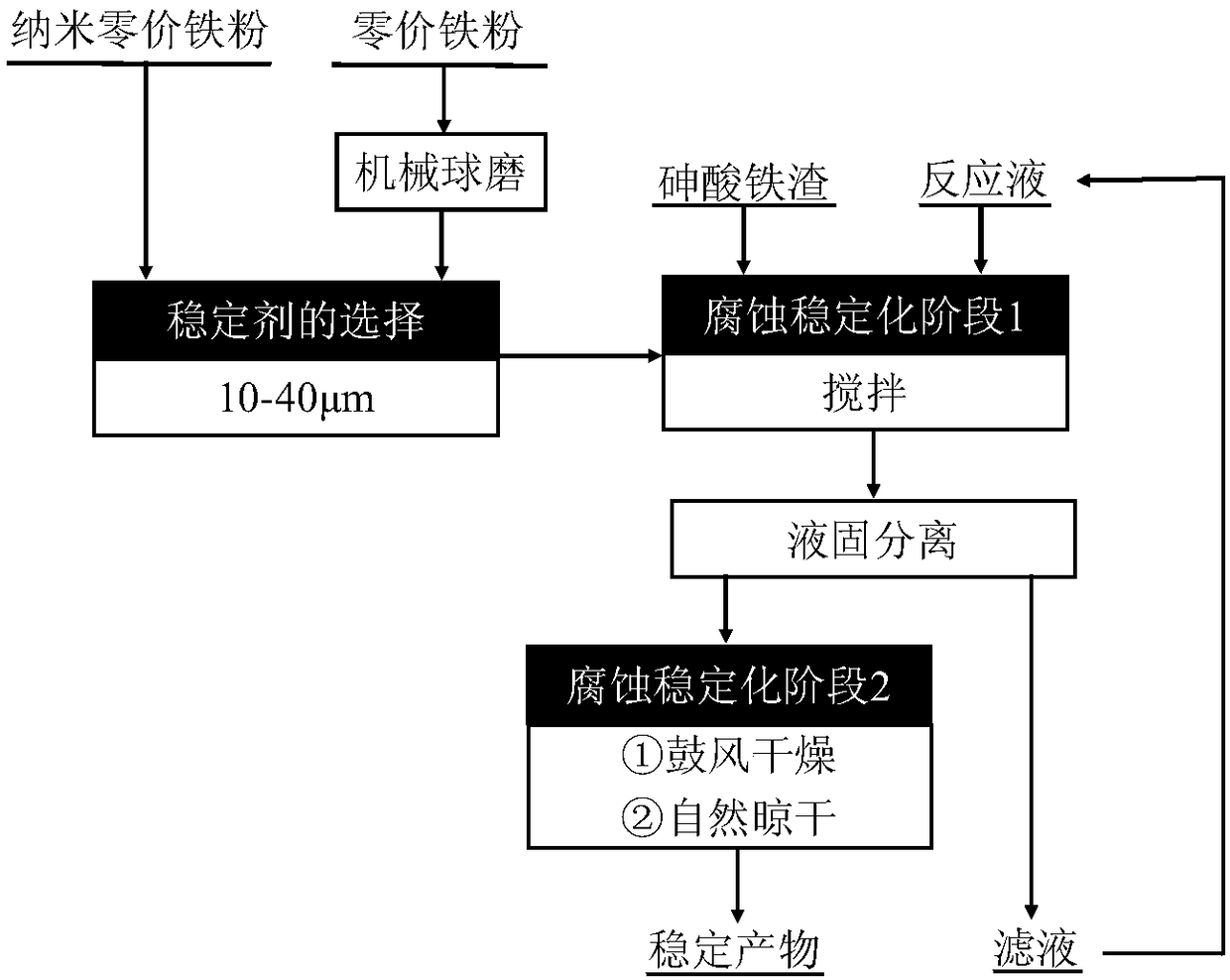
[0037] The method for stabilizing ferric arsenate slag of the present invention, concrete operation is as follows:
[0038] The stabilizer is zero-valent iron powder after ball milling, and the zero-valent iron powder is ball-milled for 1 hour to obtain ball-milled iron powder, the particle size of which is controlled at 35.8 μm; the iron arsenate slag comes from the product of A smelter after treating arsenic-containing wastewater, and the arsenic content is 22.12%, and the iron content is 22.6%.
[0039] Reaction solution: the concentration of acetic acid is 0.11mol / L, the concentration of chloride ion is 0.015mol / L (sodium chloride is added), the concentration of nitrate ion is 0.03mol / L (sodium nitrate is added), and the pH value is adjusted to 3.5 by sodium hydroxide , to obtain a reaction solution after stirring evenly.
[0040] Take 10g of ferric arsenate slag (dry basis) and 0.83g of ball-milled iron powder (the molar ratio of iron to arsenic is 0.5:1) in a conical fl...
Embodiment 2
[0043] The method for stabilizing ferric arsenate slag of the present invention, concrete operation is as follows:
[0044] The stabilizer is zero-valent iron powder after ball milling, and the zero-valent iron powder is ball-milled for 1.5 hours to obtain ball-milled iron powder, the particle size of which is controlled at 26.5 μm; the iron arsenate slag comes from the product of B smelter after treating arsenic-containing wastewater, and the arsenic The content is 15.2%, and the iron content is 15.9%.
[0045] Reaction solution: the concentration of acetic acid is 0.20mol / L, the concentration of chloride ion is 0.035mol / L (ammonium chloride is added), the concentration of nitrate ion is 0.015mol / L (ammonium nitrate is added), the pH value is adjusted to 5.7 with ammonia water, and stirred After uniformity, the reaction solution was obtained.
[0046]Take 10g of ferric arsenate slag (dry basis) and 0.57g of ball-milled iron powder (the molar ratio of iron to arsenic is 0.5:1...
Embodiment 3
[0049] The method for stabilizing ferric arsenate slag of the present invention, concrete operation is as follows:
[0050] The stabilizer is zero-valent iron powder after ball milling, and the zero-valent iron powder is ball-milled for 2 hours to obtain ball-milled iron powder, the particle size of which is controlled at 25.6 μm; iron arsenate slag is prepared by the laboratory, with arsenic content of 33.05%, iron The content is 23.07%, and it is naturally dried to a moisture content of 5%.
[0051] Reaction solution: the concentration of acetic acid is 0.10mol / L, the concentration of chloride ion is 0.025mol / L (potassium chloride is added), the concentration of nitrate ion is 0.025mol / L (potassium chloride), and the pH value is adjusted to 6.0 by potassium hydroxide. After stirring evenly, a reaction solution was obtained.
[0052] Take 10g of ferric arsenate slag (dry basis) and 1.24g of ball-milled iron powder (the molar ratio of iron to arsenic is 0.5:1) in a conical fl...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com