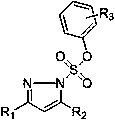
Pyrazole compound containing N-aryl sulfonate and synthesis and application thereof
A technology of aryl sulfonate and compound, applied in medical preparations containing active ingredients, organic chemistry, organic active ingredients, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Embodiment 1, the synthesis of phenyl-5-methyl-3-p-tolyl-1-hydropyrazole-1-sulfonate
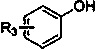
[0046] (1) Preparation of phenylsulfonyl fluoride: take a 100ml round bottom flask, add a magnet, add phenol (9.4g, 100mmol, 1.0eq) and 50ml of dichloromethane, then add triethylamine (20.8ml, 150mmol, 1.5eq), was introduced into sulfuryl fluoride gas, reacted at room temperature for 2-6h, monitored by TLC plate, extracted with dichloromethane after the reaction was complete, and the solvent was spin-dried to obtain phenylsulfonyl fluoride. Its structural formula and data representation are as follows:
[0047]
[0048] 1 H NMR (300 MHz, CDCl 3 ) δ 7.31 – 7.48 (m, 5H). 19 F NMR (282 MHz, CDCl 3 ) δ37.38;
[0049](2) Preparation of 1-p-methylphenylbutane-1,3-dione: Take a 100ml round bottom flask, add a magnet, and add sodium hydride (1.76g, 44mmol, purity 60%, 1.1eq) into it , then add 50ml of ethyl acetate under ice bath, slowly add p-methylacetophenone (5.37g, 40mmol, 1.0eq...
Embodiment 2
[0058] Embodiment 2, the synthesis of p-iodophenyl-5-methyl-3-p-tolyl-1-hydropyrazole-1-sulfonate
[0059] (1) Preparation of p-iodophenylsulfonyl fluoride: Take a 100ml round bottom flask, add a magnet, add p-iodophenol (4.4g, 20mmol, 1.0eq) and 50ml of dichloromethane, and then add triethylamine (4.2ml, 30mmol, 1.5eq), pass through sulfuryl fluoride gas, react at room temperature for 2-6h, monitor with TLC plate, extract with dichloromethane after the reaction is complete, spin the solvent to obtain p-iodophenylsulfonyl fluoride . Its structure and characterization data are as follows:
[0060]
[0061] 1 H NMR (300 MHz, CDCl 3 ) δ 8.40 (d, J = 7.8 Hz, 2H), 7.56 (d, J = 7.8 Hz,2H). 19 F NMR (282 MHz, CDCl 3 ) δ 37.35;
[0062] (2) Preparation of 1-p-tolylbutane-1,3-dione: same as Example 1;
[0063] (3) Preparation of 3-methyl-5-p-tolyl 1-hydropyrazole: same as Example 1;
[0064] (4) Preparation of p-iodophenyl-5-methyl-3-p-tolyl-1-hydropyrazole-1-sulfonate: ...
Embodiment 3
[0067] Example 3, Preparation of o-iodophenyl-5-methyl-3-p-tolyl-1-hydropyrazole-1-sulfonate
[0068] (1) Preparation of o-iodophenylsulfonyl fluoride: Take a 100ml round bottom flask, add a magnet, add o-iodophenol (11.0g, 50mmol, 1.0eq) and 50ml of dichloromethane, and then add triethylamine (13.9ml, 100mmol, 2.0eq), add sulfuryl fluoride gas, react at room temperature for 2-6h, monitor with TLC plate, extract with dichloromethane after the reaction is complete, and spin to dry the solvent to obtain o-iodophenylsulfonyl fluoride ester;
[0069] (2) Preparation of 1-p-tolylbutane-1,3-dione: same as Example 1;
[0070] (3) Preparation of 3-methyl-5-p-tolyl 1-hydropyrazole: same as Example 1;
[0071] (4) Synthesis of o-iodophenyl-5-methyl-3-p-tolyl-1-hydropyrazole-1-sulfonate: take a 50ml round bottom flask, add magnetons, and add 3-methyl - 5-p-tolyl-1-hydropyrazole (1.72g, 10mmol, 1.0eq) and o-iodophenylsulfonyl fluoride (4.4g, 20mmol, 2.0eq), then potassium tert-butoxide...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com