Preparation method of ketoxime
A ketoxime and azole-based technology, applied in the field of preparation of ketoxime, can solve the problems of equipment and environmental impact, large consumption of alkali, large consumption, etc., achieve mild reaction conditions, high product yield and purity, and increase affinity. Effects of nuclear capabilities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] In a three-necked flask, mix benzophenone and hydroxylamine hydrochloride at a molar ratio of 1:1.1-1:1.3, add an appropriate amount of ethanol and stir to completely dissolve it, and then add a certain amount of [P 4444 ][4-CH 3 Im] (the amount of the substance is 10%-50% of the hydroxylamine hydrochloride), heating to reflux, and using thin-layer chromatography (developing solvent: petroleum ether: ethyl acetate = 4:1) to monitor the reaction process. After the reaction, the solvent ethanol was removed by rotary evaporation, an appropriate amount of deionized water was added and stirring was continued, the solid was precipitated, filtered and washed with a small amount of deionized water to obtain a white solid, which was dried under vacuum at 60°C for 24 hours, and the crude yield was 90%; Chromatography (mobile phase acetonitrile:water=7:3, UV detection wavelength at 254nm) determined the purity to be 98% (area normalization method). The structure of benzophenone oxim...
Embodiment 2- Embodiment 23
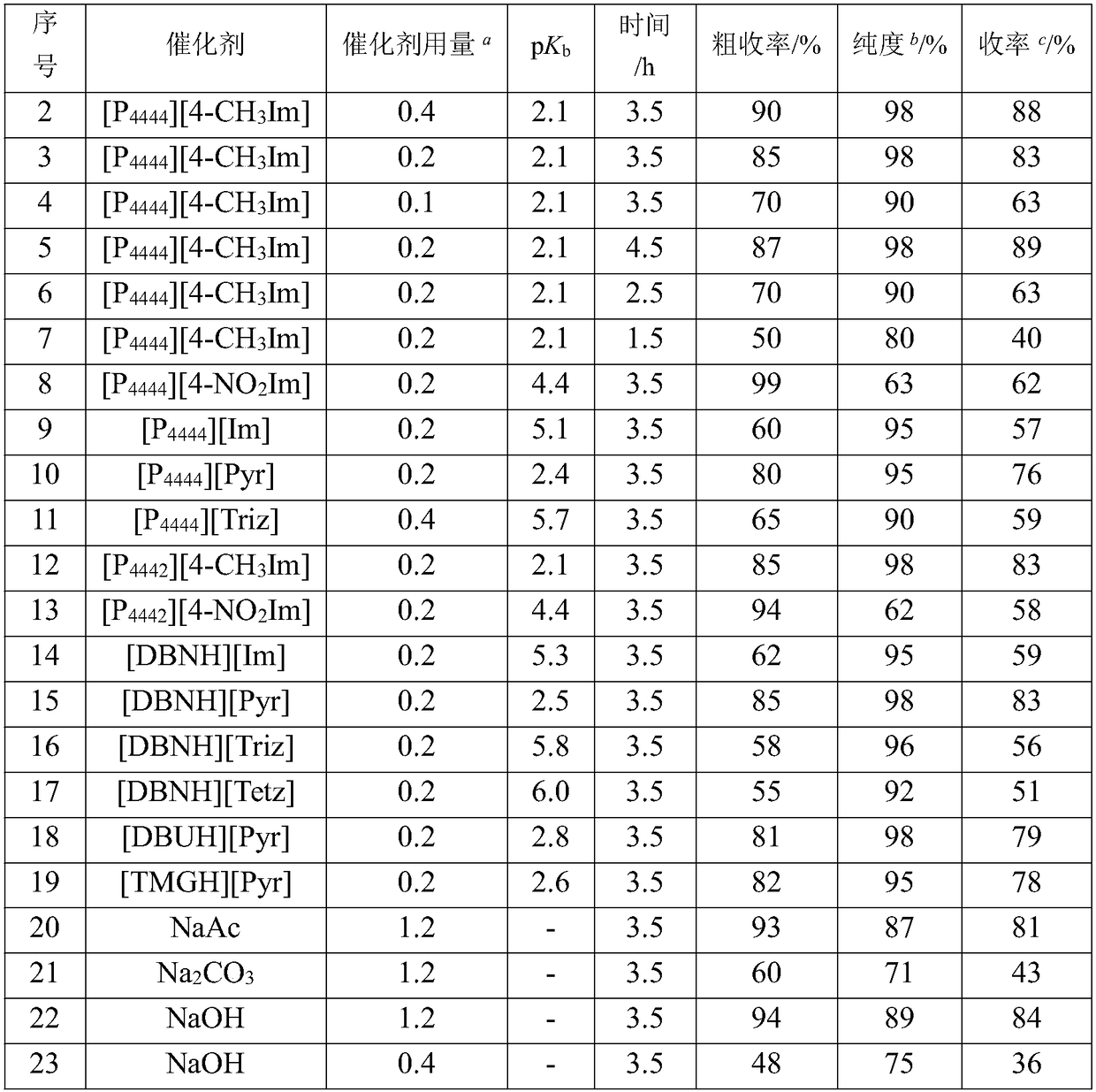
[0030] The same steps as in Example 1 are used, except for the type and amount of catalyst and the basicity of the oxazolyl anion functionalized ionic liquid, as shown in the following table:
[0031]
[0032] a Compared with the amount of hydroxylamine hydrochloride substance; b HPLC detection, mobile phase acetonitrile: water = 7:3, area normalization method; c It is obtained by multiplying the purity and crude yield.
[0033] As shown in the table, the azole-based anion functionalized ionic liquids all show strong alkalinity, which efficiently catalyzes the reaction of benzophenone and hydroxylamine hydrochloride to prepare benzophenone oxime, and has both the functions of acid binding agent and dehydrating agent. The stronger the basicity, the better the performance of catalyzing the reaction of ketone and hydroxylamine hydrochloride to prepare ketoxime. At the same time, it is found that the alkalinity of the azole-based anion functionalized ionic liquid is mainly related to t...
Embodiment 24
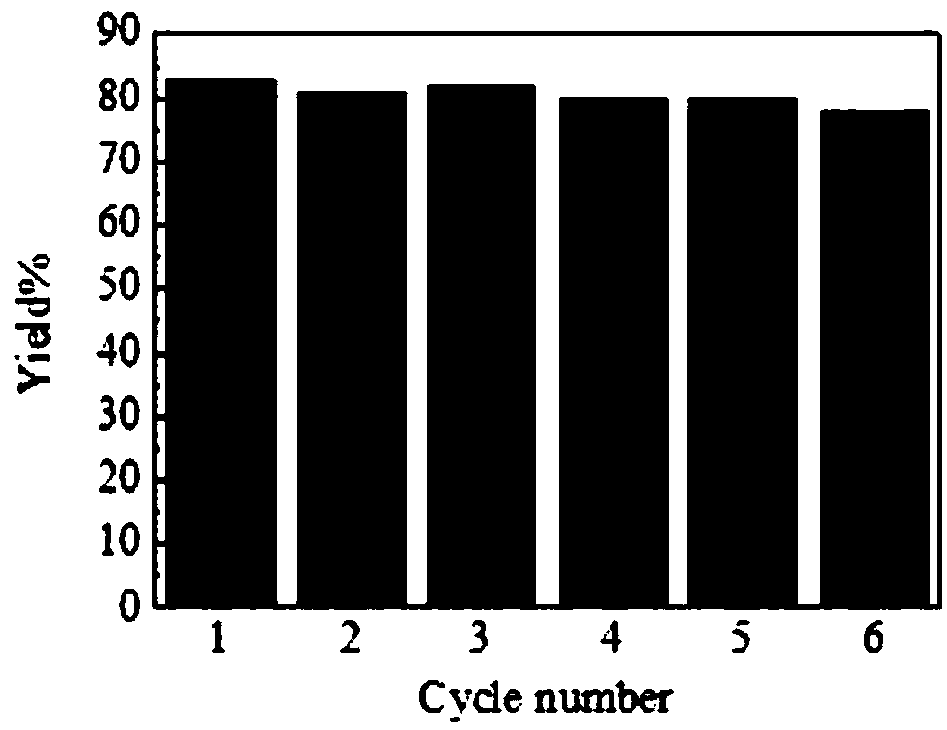
[0034] Example 24 Recycling performance
[0035] Take [P 4444 ][4-CH 3 Im] is the catalyst, the dosage is 20% (mole percentage) of hydroxylamine hydrochloride, the molar ratio of hydroxylamine hydrochloride: benzophenone is 1.2:1, and the reflux reaction is 3.5h with ethanol as the solvent. The yield of benzophenone oxime is the best , Up to 83%.
[0036] The filtrate obtained by separating the solid product after the above reaction was rotary evaporated to remove water and other solvents to obtain [P 4444 ][4-CH 3 Im] Ionic liquid, washed 3 times with ethyl acetate, dried in vacuum at 50°C for 6 hours, and used as a catalyst again to prepare ketoxime by reacting benzophenone with hydroxylamine hydrochloride. After 6 cycles of use, the catalytic effect did not decrease significantly, such as figure 1 Shown.
[0037] In the traditional hydroxylamine hydrochloride to prepare ketoxime, due to NaOH and Na 2 CO 3 Other substances will neutralize with hydrochloric acid, thus losing the p...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com