C13 deuterated methyl telmisartan and preparation method and application thereof
A technology of telmisartan and methyl substitution, which is applied in the field of carbon 13 deuterated methyl telmisartan and its preparation, and achieves the effects of high yield, reduced production cost, and reduced death
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
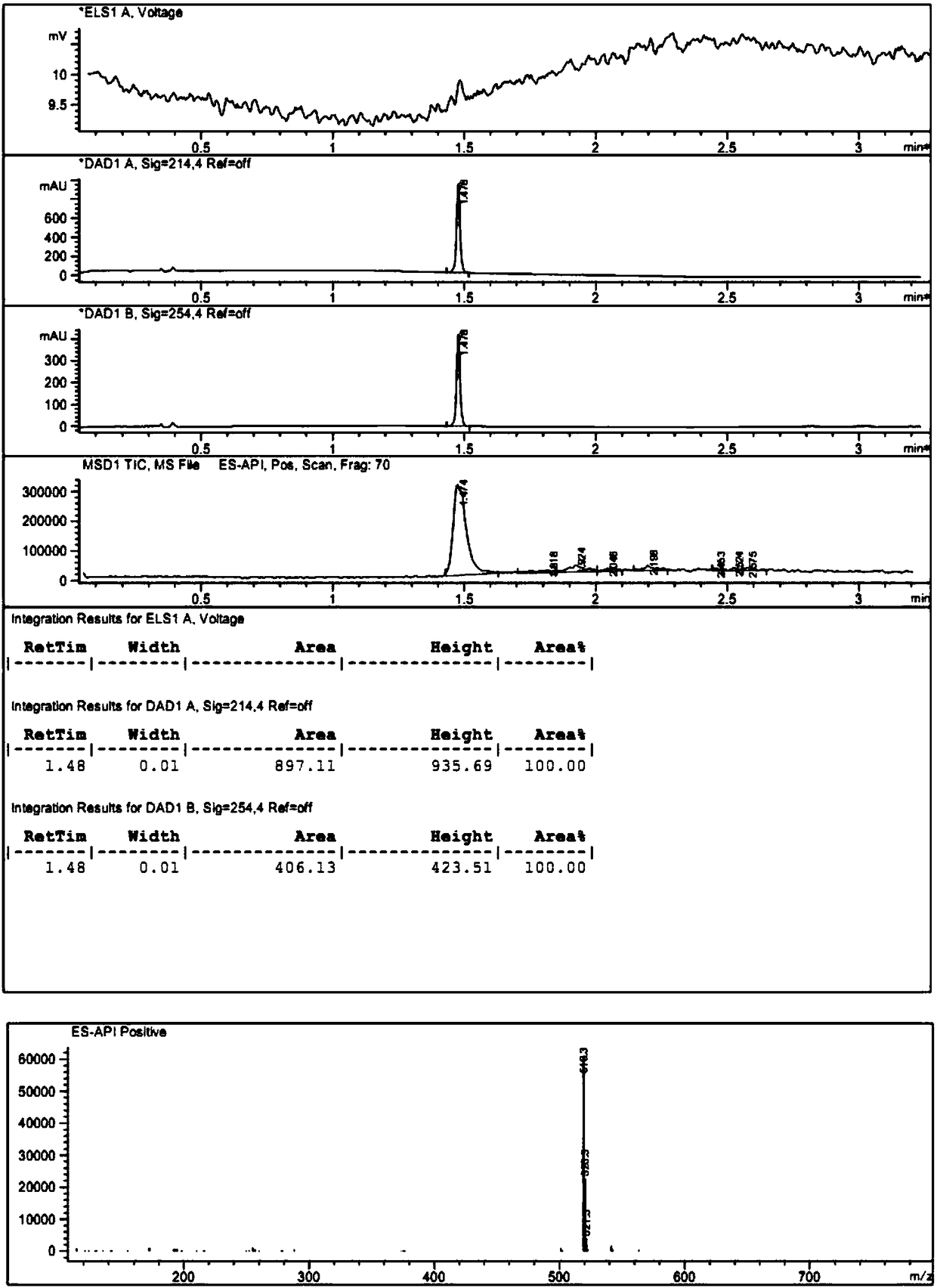
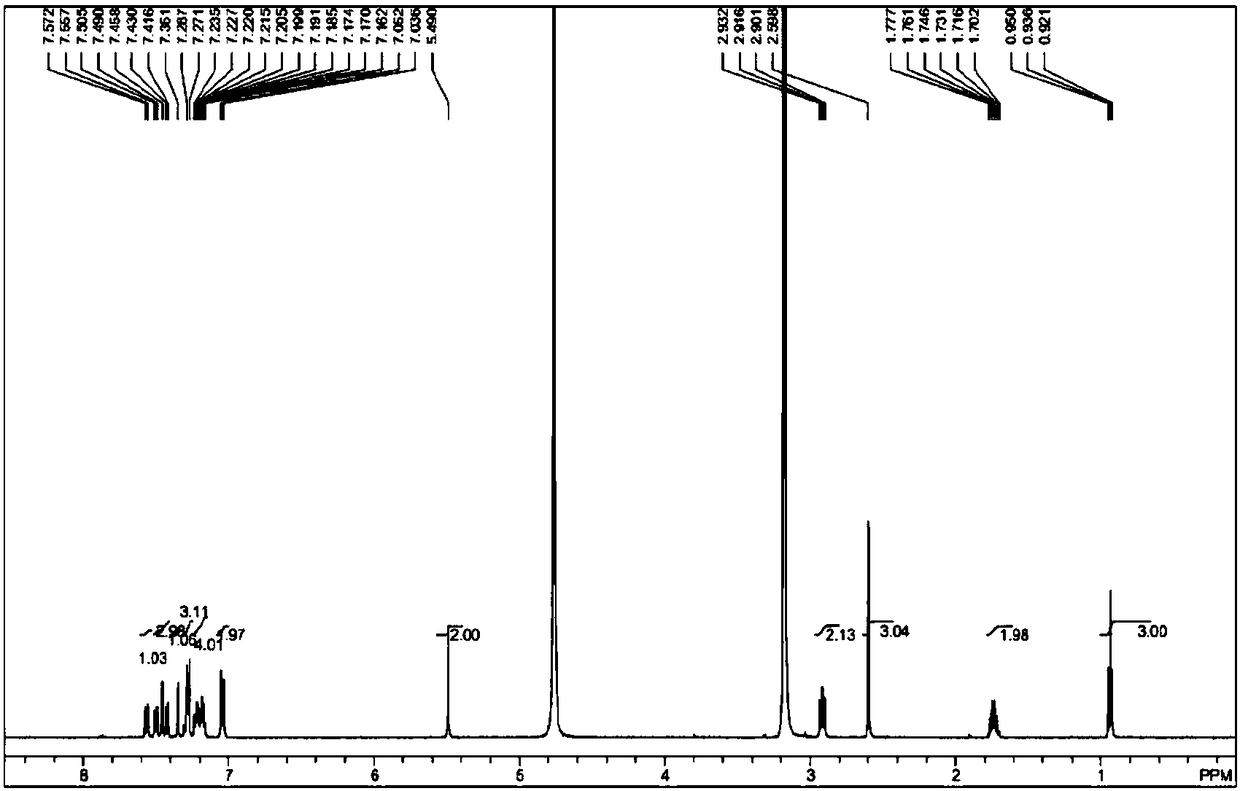
Image
Examples
preparation example Construction
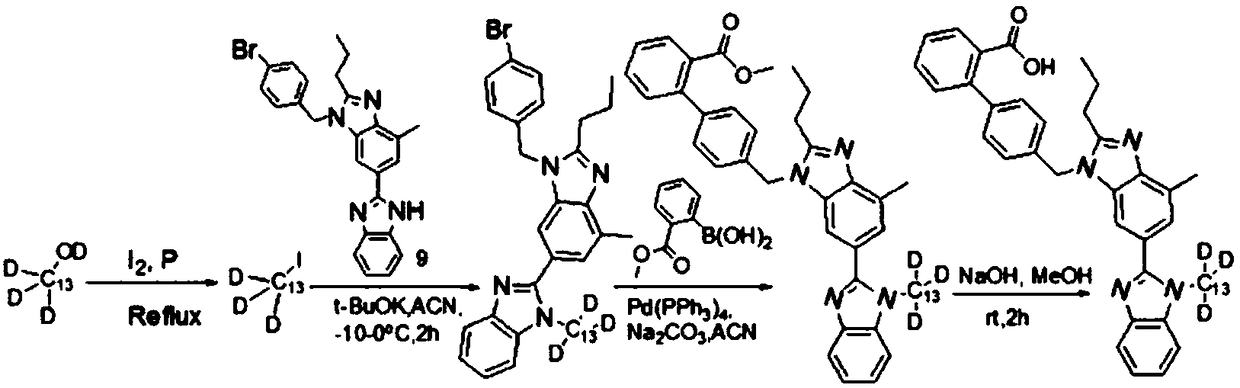
[0038]A kind of carbon 13 deuterium replaces the preparation method of misartan, it comprises the following steps:
[0039] 1): Mix carbon-13 deuterated methanol and iodine, and catalyze carbon-13 deuterated methyl iodide by refluxing red phosphorus (Ⅱ)
[0040]
[0041] 2): Dissolving compound (Ⅲ) in a solvent, adding base, and adding carbon 13 deuterated methyl iodide to react to obtain compound (Ⅳ)
[0042]
[0043] 3): Dissolving compound (Ⅳ) in a solvent, adding reaction base and catalyst, and preparing compound (Ⅴ) through Suzuki coupling reaction
[0044]
[0045] 4): Dissolving compound (Ⅴ) in a solvent, adding a reaction base, and obtaining carbon-13 deuterium instead of misartan through hydrolysis reaction
[0046]
[0047] As a preferred version, the above-mentioned deuterium replaces misartan-C 13 d 3 The preparation method, in the reaction described in step 1, the reaction mass ratio of carbon-13 deuterated methanol to iodine and red phosphorus is 1...
Embodiment 1
[0054] Step 1, the preparation of compound (II):
[0055]
[0056] Mix iodine element (19.5g) with carbon-13 deuterated methanol (5.0g) and place it in a 150mL constant pressure dropping funnel. , first drop a small amount of deuterated methanol mixture that dissolves part of the iodine in the constant pressure dropping funnel into the round bottom flask, then heat to reflux, and when the temperature rises to about 50°C, drop the remaining mixed solution into the round bottom flask in batches in the flask. The dropwise addition was completed in about 30 minutes. The reflux continued to dissolve the iodine and drop it in. After all the iodine in the dropping funnel was dissolved, stop heating and cool to room temperature. After stirring at room temperature for 1 h, distill at atmospheric pressure, collect fractions at 41 to 44 ° C, and use saturated Na 2 S 2 o 3 Wash 3 times, wash 2 times with saturated brine, add anhydrous CaCl 2 After drying, 13.8 g of carbon-13 deut...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com