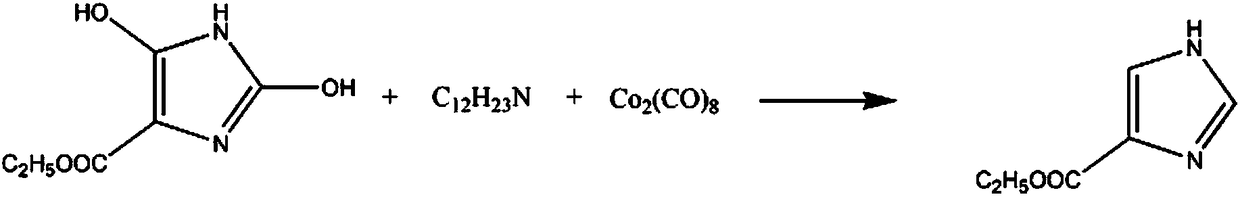Synthetic method for anti-leptospira drug ethyl imidazolate
A technology of imidazolate ethyl ester and a synthetic method, which is applied in the direction of organic chemistry, can solve the problems of high equipment corrosion resistance, increased production cost, complex process, etc., and achieve the reduction of equipment corrosion resistance and low production cost. The effect of reducing and increasing the reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] The synthetic method of anti-leptospirosis drug ethyl imidazole comprises the steps:
[0016] A: Add 2mol ethyl 2,5-dihydroxyimidazole-4-carboxylate and 1.9L potassium sulfate solution with a mass fraction of 15% in the reaction vessel, control the stirring speed to 210rpm, increase the temperature to 30°C, and continue the reaction for 90min ;
[0017] B: Raise the temperature to 40°C, add 6mol dicyclohexylamine solution with a mass fraction of 30%, add 3mol octacarbonyl dicobalt powder in 4 times, continue the reaction for 2h, lower the temperature to 10°C, precipitate solid, use 1.2L Wash 5 times with 10% sodium nitrate solution, 3 times with 40% cyclohexane solution, recrystallize in 80% tripropylene glycol monomethyl ether solution, dehydrating agent anhydrous magnesium sulfate After dehydration, 240.8 g of the finished imidazole ethyl ester was obtained, with a yield of 86%.
Embodiment 2
[0019] The synthetic method of anti-leptospirosis drug ethyl imidazole comprises the steps:
[0020] A: Add 2mol ethyl 2,5-dihydroxyimidazole-4-carboxylate and 1.9L potassium sulfate solution with a mass fraction of 18% in the reaction vessel, control the stirring speed at 230rpm, raise the temperature to 33°C, and continue the reaction for 110min ;
[0021] B: Raise the temperature to 42.5°C, add 7mol mass fraction of 33% dicyclohexylamine solution, add 3.5mol octacarbonyl dicobalt powder in 5 times, continue the reaction for 2.5.h, lower the temperature to 12.5°C, and precipitate a solid, Wash 6 times with 1.2L of 13.5% sodium nitrate solution, 4 times with 42.5% cyclohexane solution, recrystallize in 83% tripropylene glycol monomethyl ether solution, dehydrating agent activity Alumina was dehydrated to obtain 254.8 g of imidazole ethyl ester with a yield of 91%.
Embodiment 3
[0023] The synthetic method of anti-leptospirosis drug ethyl imidazole comprises the steps:
[0024] A: Add 2mol ethyl 2,5-dihydroxyimidazole-4-carboxylate and 1.9L potassium sulfate solution with a mass fraction of 21% in the reaction vessel, control the stirring speed at 250rpm, raise the temperature to 36°C, and continue the reaction for 130min ;
[0025] B: Raise the temperature to 45°C, add 8 mol of dicyclohexylamine solution with a mass fraction of 36%, add 4 mol of dicobalt octacarbonyl powder in 6 times, continue the reaction for 3 hours, lower the temperature to 15°C, precipitate a solid, and use 1.2L Wash 7 times with 17% sodium nitrate solution, 5 times with 45% cyclohexane solution, recrystallize in 86% tripropylene glycol monomethyl ether solution, dehydrating agent anhydrous magnesium sulfate After dehydration, 263.2 g of the finished imidazole ethyl ester was obtained, with a yield of 94%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com