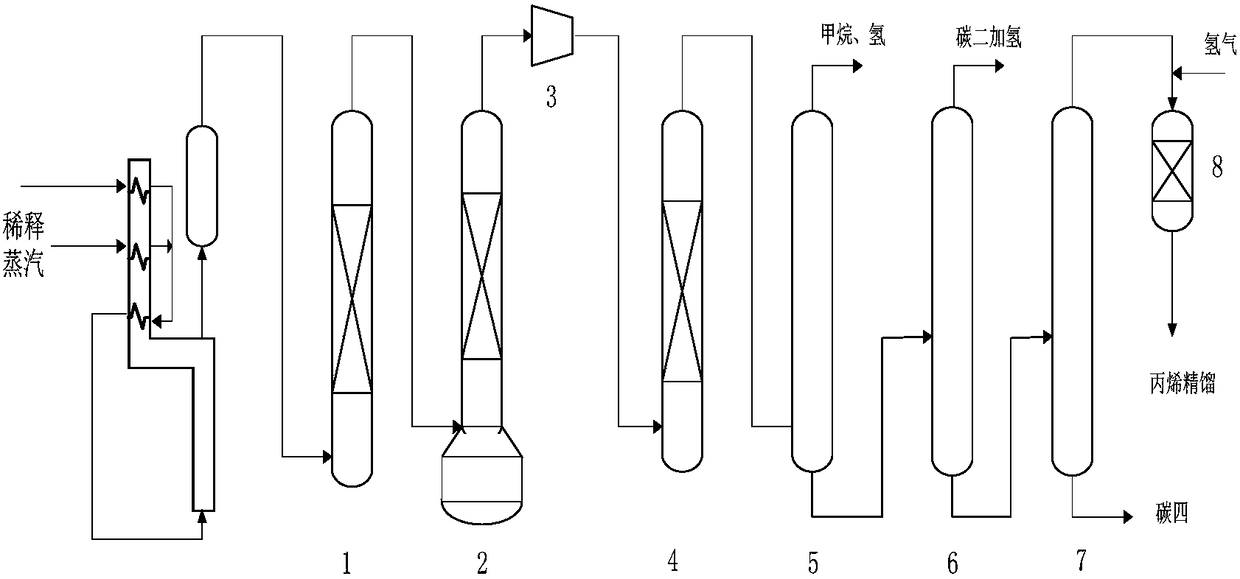
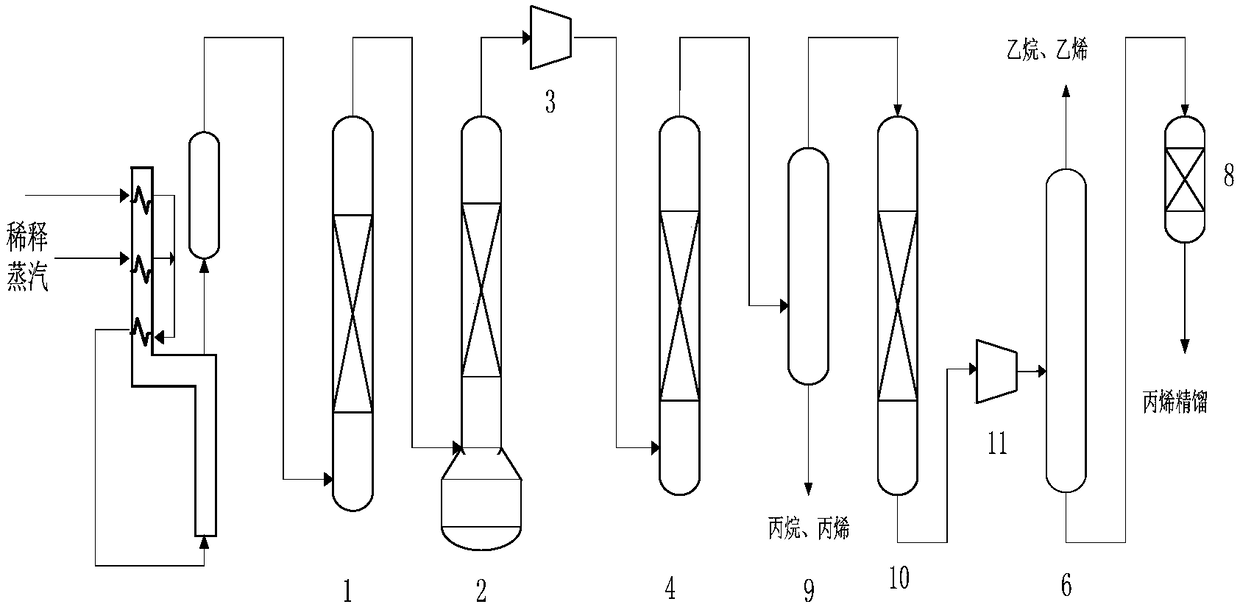
Selective hydrogenation method for C3 fraction
A selective hydrogenation and fractionation technology, applied in hydrocarbons, chemical instruments and methods, purification/separation of hydrocarbons, etc., can solve problems such as long distances in industrial applications, and achieve excellent anti-coking performance and operational flexibility Good, no ethylene loss effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] Weigh a clover-shaped alumina carrier of Φ4.5×4.5mm. Take ferric nitrate, dissolve it in 60ml of deionized water by heating, adjust the pH value to 2.5, impregnate the equal volume on the surface of the carrier at the temperature of 50°C, flip the carrier quickly for 6 minutes, let it stand still for 30 minutes until the adsorption equilibrium, and age at 60°C for 30 minutes, then in In the oven follow the procedure: Dry the catalyst, and then use the temperature programming method to activate the catalyst. The activation procedure: Weigh cobalt nitrate and impregnate according to the above preparation steps. The physical properties of the carrier and catalyst and the content of each component of the catalyst are shown in Table 1.
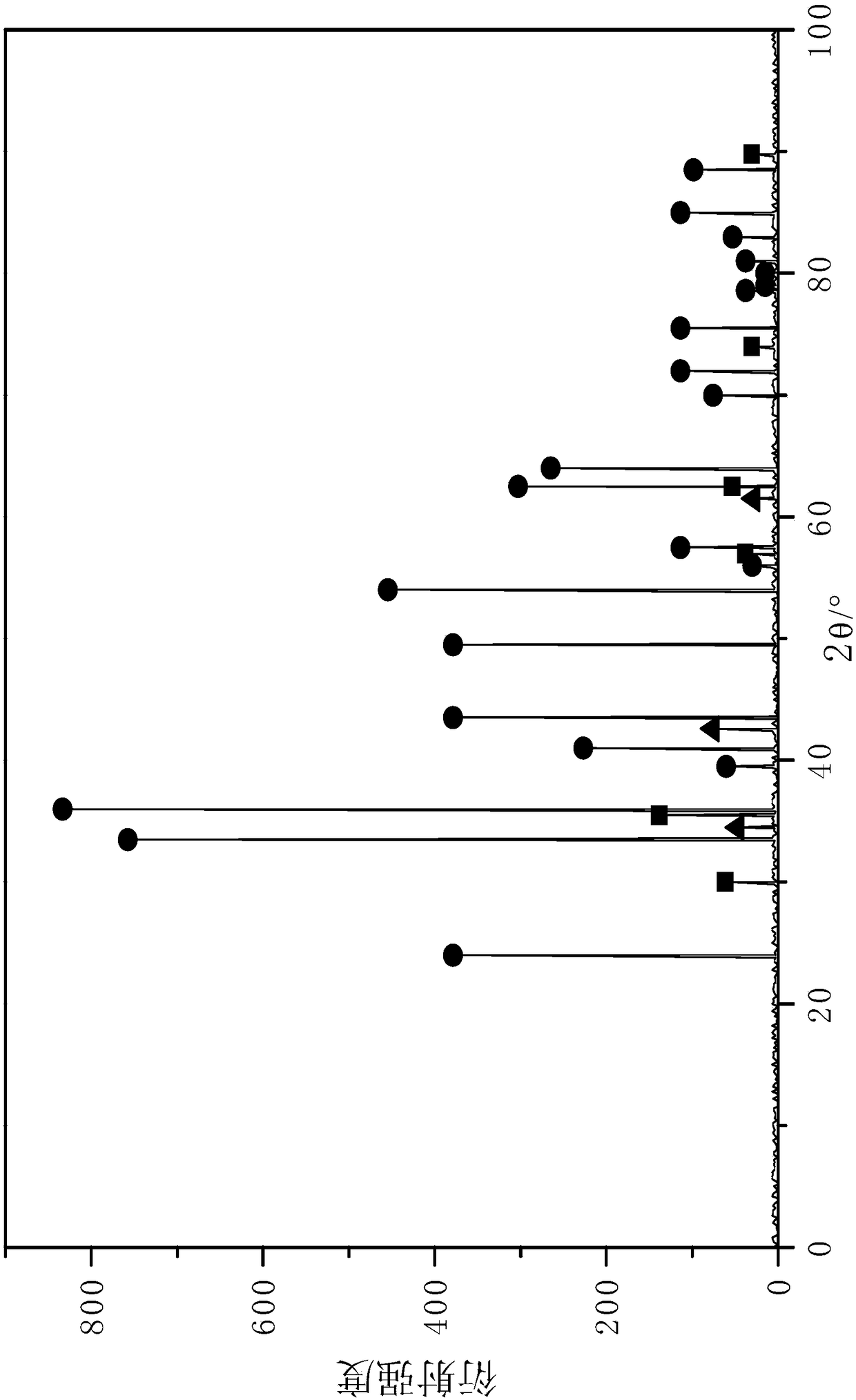
[0071] Before the catalyst is used, it is reduced with 40% hydrogen + 60% nitrogen in a reduction furnace, the reduction temperature is 420° C., the pressure is 0.5 MPa, and the reduction time is 4 hours. Catalyst XRD analysis after r...
Embodiment 2
[0074] At 50°C, the NaAlO 2 solution and ZrCl 4 The solution was stirred and mixed, then neutralized with nitric acid solution, stirred for 10 hours, and uniform Al-Zr particles were formed by co-precipitation. The resultant was filtered and the Na in it was washed with deionized water + and Cl - Ions, and then add polyvinyl alcohol with a mass concentration of 15% as a pore-forming agent, kneading and molding. Dry at 130°C for 2h, and calcined at 650°C for 4h to obtain a Zr-Al composite support. The mass ratio of alumina to zirconia in the carrier is 4:1.
[0075]The catalyst was prepared with alumina-zirconia composite carrier. Take ferric chloride and cobalt chloride, heat and dissolve them in deionized water, adjust the pH value to 2.0, impregnate the excess on the carrier at a temperature of 80°C, shake the beaker for 10 minutes, filter off the excess impregnating liquid, and place the catalyst in a water bath at 60°C Medium aging for 50min, then follow the procedur...
Embodiment 3
[0081] Weigh 100ml of a Φ1.5mm spherical α-alumina carrier. Dissolve ferric nitrate in 40ml of deionized water, adjust the pH value to 3.0, soak the liquid at 40°C, spray it on the carrier with a watering can, load it in the drum for 10 minutes to make the active components evenly loaded, and control the loading process in 6 minutes to complete, and then In the oven follow the procedure: Dry the catalyst, move the catalyst into an evaporating dish, and activate the catalyst in a muffle furnace using a temperature-programmed method. Activation procedure: room temperature Obtain a catalyst dip.
[0082] Using the same method as the first step, take cobalt nitrate, dissolve it, spray it on the surface of the catalyst, dry it, and roast it to obtain the final catalyst. Drying procedure: Roasting procedure:
[0083] The physical properties of the carrier and catalyst and the content of each component of the catalyst are shown in Table 1.
[0084] Before the catalyst i...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Specific surface area | aaaaa | aaaaa |
| Pore volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com