Electrode for selectively adsorbing noble metal and method for selectively recycling noble metal
A precious metal and selective technology, applied in the direction of improving process efficiency, can solve the problems of unsuitable metal recovery from wastewater, low concentration, and many types of metal ions, and can shorten the gold adsorption time, increase the amount of adsorption, and improve the adsorption efficiency. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0047] A.Materials and methods
[0048] 1. Strain activation and proliferation culture
[0049] Microthermophilic B-type Proteus (Tepidimonas fonticaldi sp.nov.) BCRC 80391 (November 21, 2011, publicly deposited in Bioresource Collection and Research Center (BCRC), Taiwan Food Industry Development Institute, China , this bacterium was also publicly deposited in the Belgian Culture Collection Center (Laboratorium voor. Microbiologie Gent'Belgium; LMG) on November 28, 2011, and its deposit number is LMG26746. Take it out of the library. 500 μL of frozen bacteria liquid was implanted into 50 mL of 1 / 5 TSB (Trypcase Soy Broth) liquid medium, and activated at a shaking rate of 180 rpm and 55°C for 24 hours. After the activation of the strain was completed, 1 mL of the activated bacterial liquid was implanted into 100 mL of liquid medium, and cultured for 2 days at a shaking rate of 180 rpm and 55° C. to obtain a fermentation liquid.
[0050] Afterwards, the fermentation broth wa...
preparation example 1
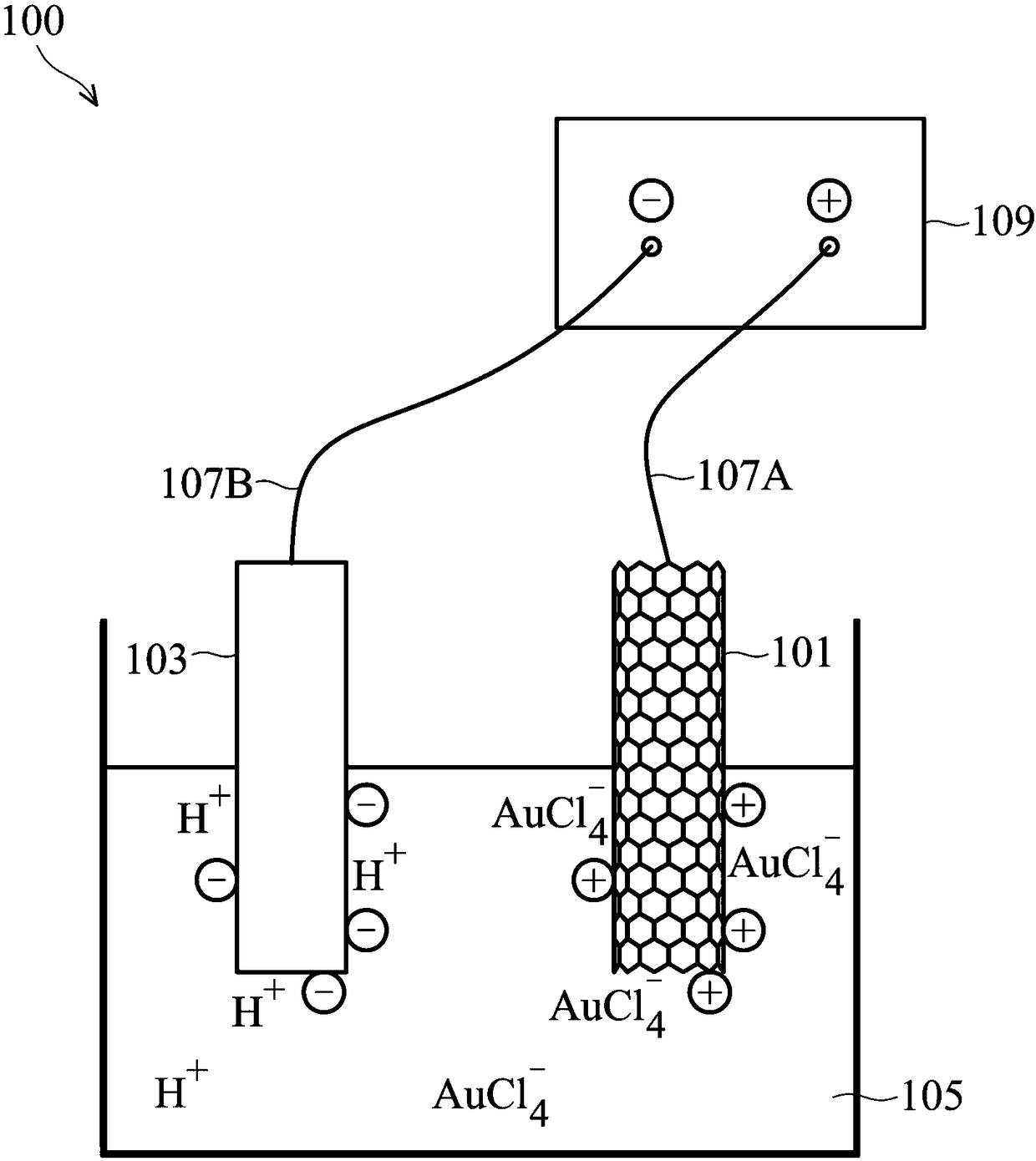
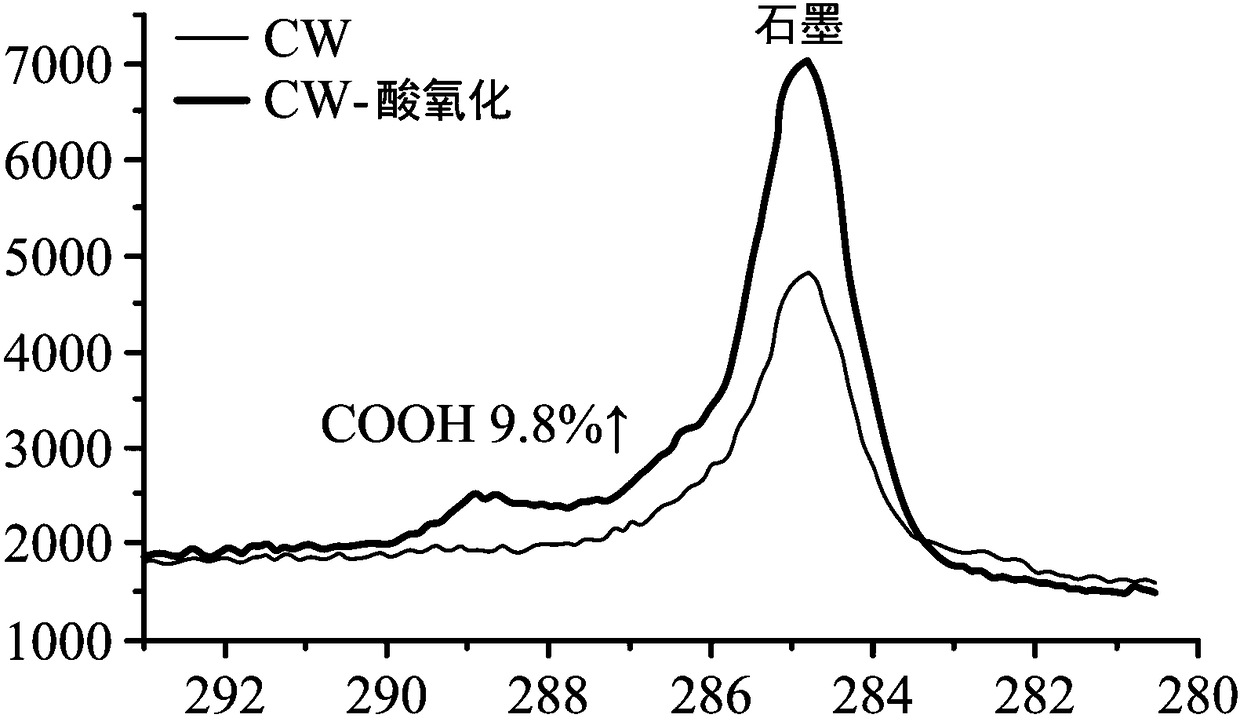
[0067] Cut the conductive heating carbon fiber cloth (CW) into 2*2cm 2 size.
[0068] After that, the carbon fiber cloth is subjected to acid oxidation, and the steps of acid oxidation are as follows.
[0069] First the carbon fiber cloth is washed with acetone. Next, the carbon fibers were placed in a 3M nitric acid solution and heat-treated at 70°C for 24 hours. The purpose of this step was to increase the carboxyl groups (-COOH) on the surface of the carbon fiber cloth. Afterwards, the carbon fiber cloth was washed with deionized water.
[0070] After washing, the carbon fiber cloth was soaked in 30 mL of protein suspension, and shaken at room temperature at 150 rpm for 24 hours, and then washed twice with deionized water (10 mL) to obtain a protein-immobilized carbon material electrode.
preparation example 2
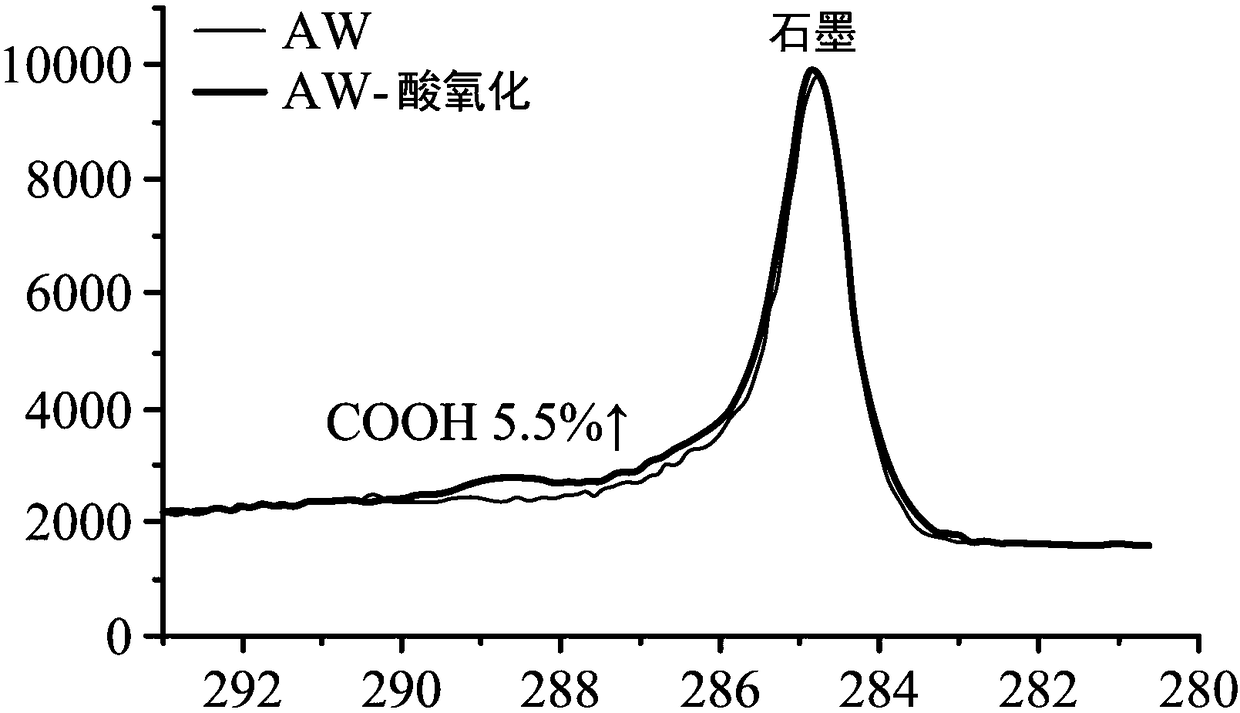
[0072] Cut the conductive heating carbon fiber cloth (CW) into 2*2cm 2 size.
[0073] After that, the carbon fiber cloth is subjected to acid oxidation, and the steps of acid oxidation are as follows.
[0074] First the carbon fiber cloth is washed with acetone. Next, arrange the carbon fibers in a 3M nitric acid solution and heat-treat at 70°C for 24 hours. The purpose of this step is to increase the carboxyl group (-COOH) on the surface of the carbon fiber cloth to enhance the amidation effect on the surface of the carbon fiber cloth. Afterwards, the carbon fiber cloth was washed with deionized water.
[0075] After washing, the carbon fiber cloth is amidated, and the amidation steps are as follows.
[0076]The carbon fiber cloth was soaked in N-hydroxysuccinimide (N-hydroxysuccinimide, NHS) solution (as amidated coupling agent) (10mL; 50mg / mL; pH 2.99) and 2-(N-morpholine) ethanesulfonate Acid (2-Morpholino-ethanesulfonic acid, MES) buffer solution (10mL; 0.001M; pH 4.5...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com