Recombinant arginine deiminase, industrial preparation method and applications
An arginine deiminase and pre-purification technology, which is applied in the field of industrial preparation, recombinant arginine deiminase and its encoding gene, can solve the problem of reducing the specific activity rate of recombinant protein, unqualified product quality and precipitation. Precipitation and other problems, to achieve the effect of easy amplification and repetition, reduction of content, and clear composition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] Example 1 rADI gene optimization design
[0063] According to the cDNA sequence of arginine deiminase published by GenBank (GenBank accession number: BAA02571), the inventor determined the rADI gene sequence before the gene optimization of the present invention, and obtained the rADI of the present invention after codon optimization of the gene. Gene, as shown in SEQ ID NO:1.
[0064] The following is the codon optimization of rADI. The parameters before and after optimization are compared as follows:
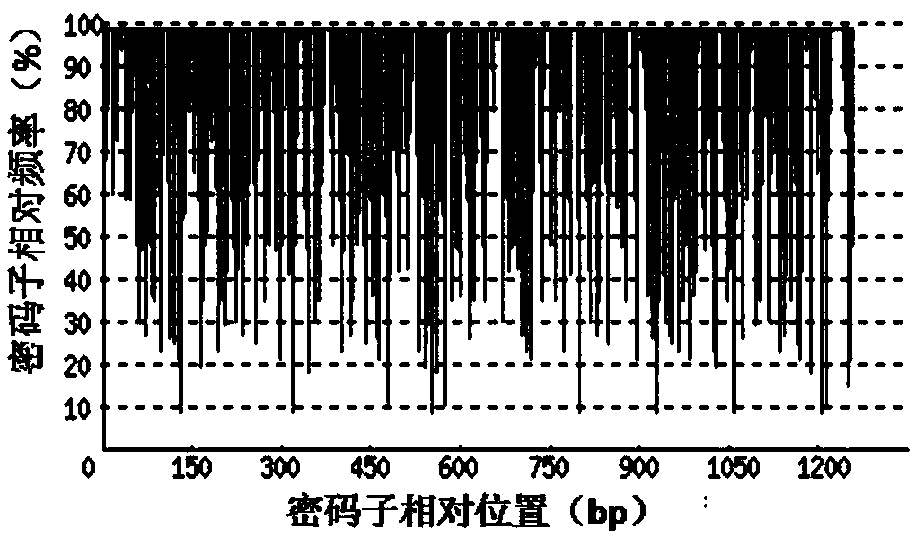
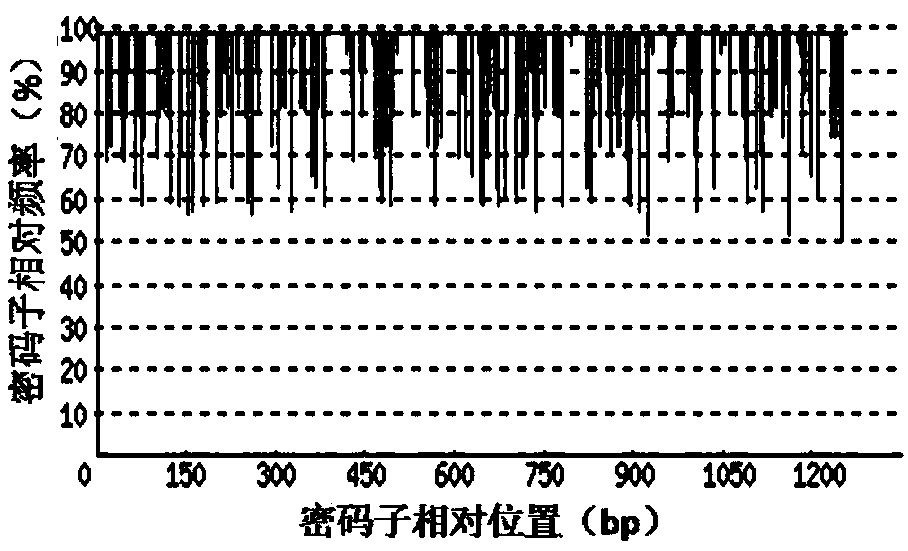
[0065] 1. Codon Adaptation Index (CodonAdaptation Index, CAI)
[0066] Depend on Figure 2-a It can be seen that before codon optimization, the codon adaptation index (CAI) of rADI natural gene in Escherichia coli was 0.60. Depend on Figure 2-b It can be seen that after codon optimization, the codon adaptation index (CAI) of the rADI-optimized gene in E. coli was 0.90. Usually, when CAI=1, it is considered that the target gene is in the most ideal high-efficiency...
Embodiment 2
[0071] Example 2 rADI strain construction and expression identification
[0072]The optimized recombinant arginine deiminase whole gene (as shown in SEQ ID NO: 1) was introduced upstream and downstream into restriction endonuclease sites Xba I and Xho I, respectively, and constructed into pUC57 plasmid, A long-term preserved cloning plasmid was obtained, which was designated as pUC57-rADI plasmid (gene synthesis and cloning, and construction of the plasmid were entrusted to Nanjing GenScript Co., Ltd.). In order to clone the rADI gene from the pUC57 vector into the pET28a vector, and then transform it into an expression host, see the following steps:
[0073] 1. Take the glycerol tube of DH5α-pET28a (preserved in our laboratory) frozen in the refrigerator at -80°C, inoculate it in LB liquid medium after thawing, and culture it overnight on a shaker at 220 rpm at 37°C. Extraction (purchased from Tiangen Biochemical Technology Co., Ltd.).
[0074] 2. Use primers to amplify t...
Embodiment 3
[0081] Example 3 Establishment of rADI Escherichia coli Tertiary Seed Bank
[0082] Step 1: Establishment of the original seed bank
[0083] 1. Pick a single colony of recombinant rADI Escherichia coli that has been verified, streak it on an LB plate containing 50 μg / mL kanamycin (0408, purchased from Amresco), and culture overnight at 37°C.
[0084] 2. Pick a single colony and inoculate it in 5 mL of LB liquid medium containing 50 μg / ml kanamycin, culture at 37°C and 220 rpm when the OD600 reaches 1.0-1.2, then stop the culture.
[0085] 3. Take 700 μl of the bacterial solution into a sterile pyrogen-free cell cryopreservation tube, then add 500 μL of 50% glycerol solution, and store it in aliquots as the original seed bank.
[0086] Step 2 Establishment of the main seed bank
[0087] 1. Streak the original seed bank bacterial liquid on the LB plate containing 50ug / mL kanamycin, culture overnight at 37°C.
[0088] 2. Pick a monoclonal colony and inoculate it in 5 mL of L...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com