Method used for separating flavonoid components, and applications thereof
A technology for flavonoids and structure separation, which is applied in the field of analytical chemistry and can solve the problems of separation, time-consuming and high detection cost of astragaloside and quercitrin.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
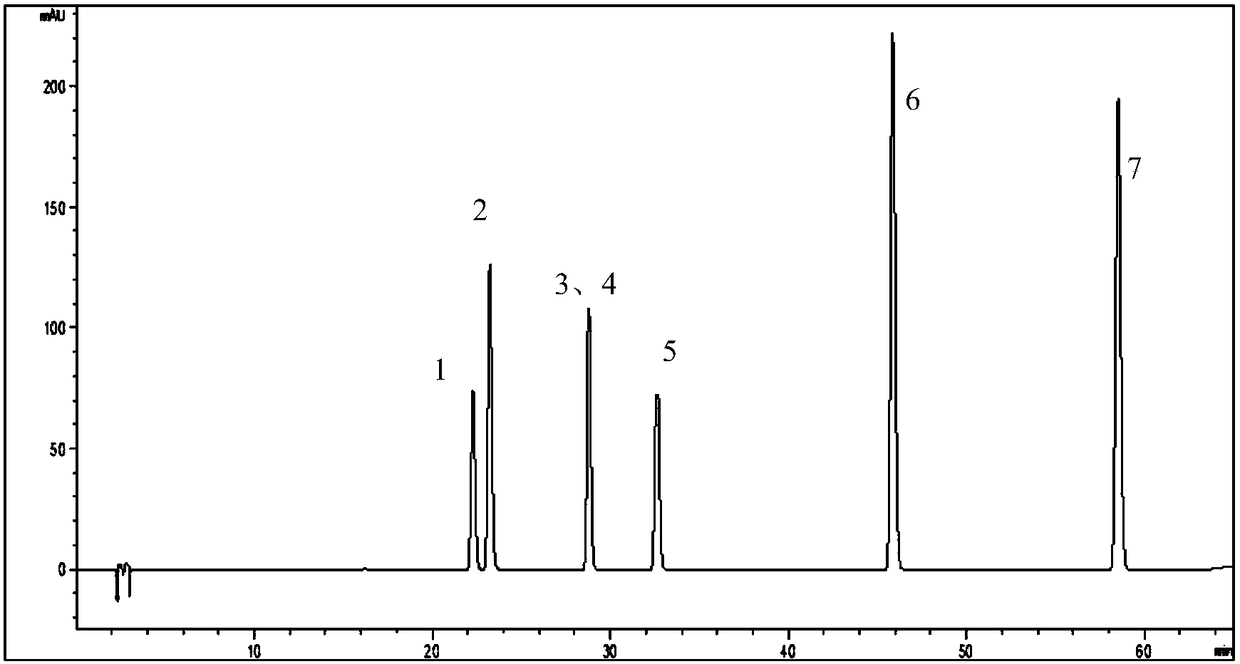
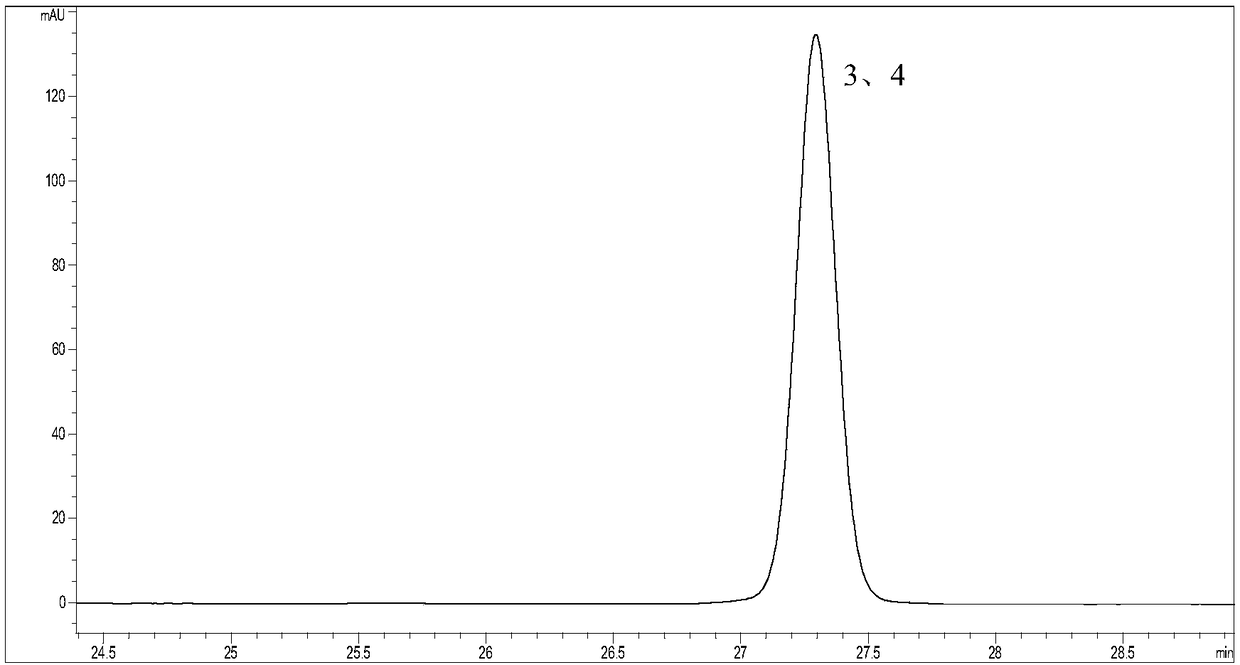
[0092] Example 1 Study on Separation of Flavonoids by High Performance Liquid Chromatography
[0093] 1. Preparation of mixed reference solution
[0094] Accurately weigh the appropriate amount of reference substances of hyperoside, isoquercitrin, astragalin, quercitrin, myricetin, quercetin, and kaempferol respectively, place them in 25mL volumetric flasks, add methanol to volume to the scale line, shake well, and formulate the mass concentrations of hyperoside 0.8116mg / mL, isoquercitrin 0.7092mg / mL, astragalin 0.7668mg / mL, quercitrin 0.7820mg / mL, myricetin 0.8332mg / mL, quercetin 0.7592mg / mL, kaempferol 0.7980mg / mL reference substance stock solution.
[0095] Take 5mL of each of the above-mentioned reference substance stock solutions, place them in the same 100mL volumetric flask, add methanol to the volume, shake well, and prepare A mixed reference solution of 0.03834 mg / mL baexin, 0.03910 mg / mL quercitrin, 0.04166 mg / mL myricetin, 0.03796 mg / mL quercetin, and 0.03990 mg...
Embodiment 2
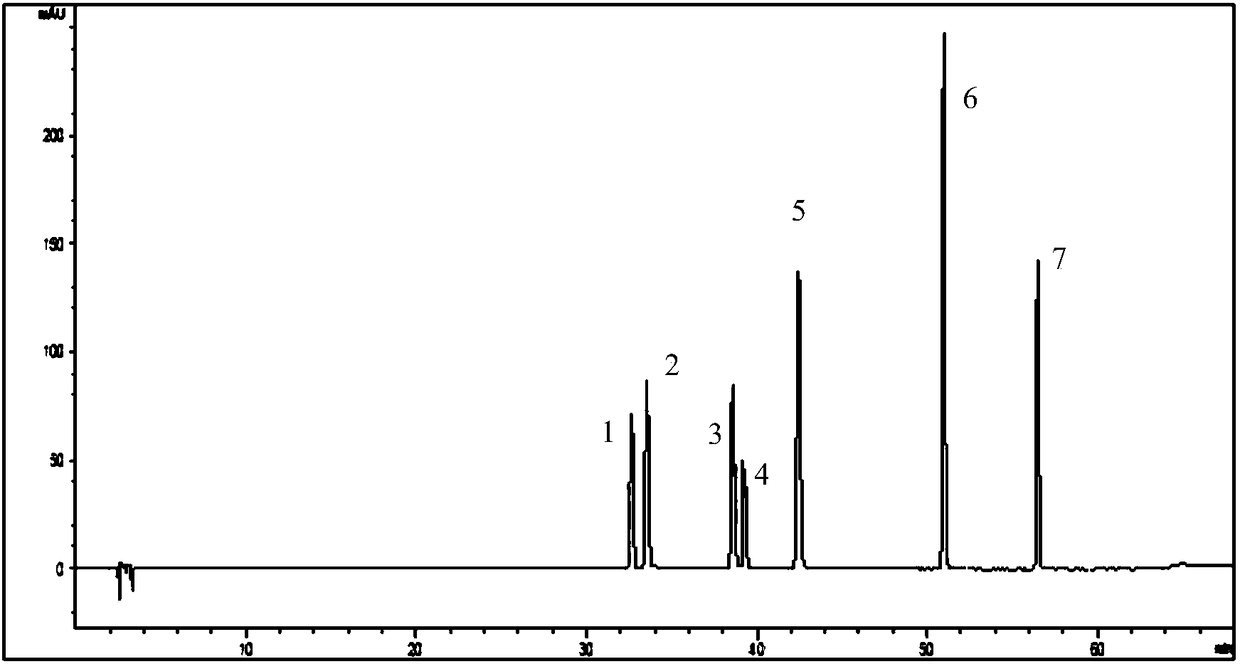
[0105] Example 2 The method established in Example 1 detects flavonoids in the persimmon leaf extract
[0106] 1. Preparation of mixed reference solution
[0107] Prepare the mixed reference substance solution according to the same method under the item "1." of Example 1.
[0108] 2. Preparation of the test solution:
[0109] Take persimmon leaf extract, take about 0.1g, weigh it accurately, put it into a stoppered Erlenmeyer flask, add 20mL of methanol precisely, seal it tightly, weigh it, ultrasonically treat it (power 250W, frequency 45kHz) for 30min, take it out, and let it cool , and then weighed, make up the lost weight with methanol, shake well, filter with a 0.45 μm microporous membrane, and take the subsequent filtrate to obtain the test solution of persimmon leaf extract.
[0110] 3. Preparation of sample for mixing and adding samples
[0111] Take the persimmon leaf extract, take 1 / 2 (about 0.05g) of the sample amount of the persimmon leaf extract test sample, ...
Embodiment 3
[0126] Example 3 Establishment of a method for determining the content of flavonoids in persimmon leaf extract based on one test and multiple evaluation
[0127] 1. Preparation of mixed reference solution
[0128] Accurately weigh the appropriate amount of reference substances of hyperoside, isoquercitrin, quercitrin, myricetin, quercetin, kaempferol and astragalin respectively, place them in a 25mL volumetric flask, add methanol to the volume Scale line, shake well, and formulate the mass concentration as hyperoside 0.8116mg / mL, isoquercitrin 0.7092 mg / mL, quercetin 0.7820mg / mL, myricetin 0.8332mg / mL, quercetin 0.7592mg / mL, the mixed reference stock solution of kaempferol 0.7980mg / mL and ascarin 0.7668mg / mL.
[0129] Accurately draw 10.0, 5.0, 3.0, 2.0, 1.0, 0.5, 0.1mL of the above-mentioned mixed reference substance stock solutions respectively, place them in a 25mL volumetric flask, dilute with methanol to the mark, and shake well to prepare a series of mixed reference su...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com