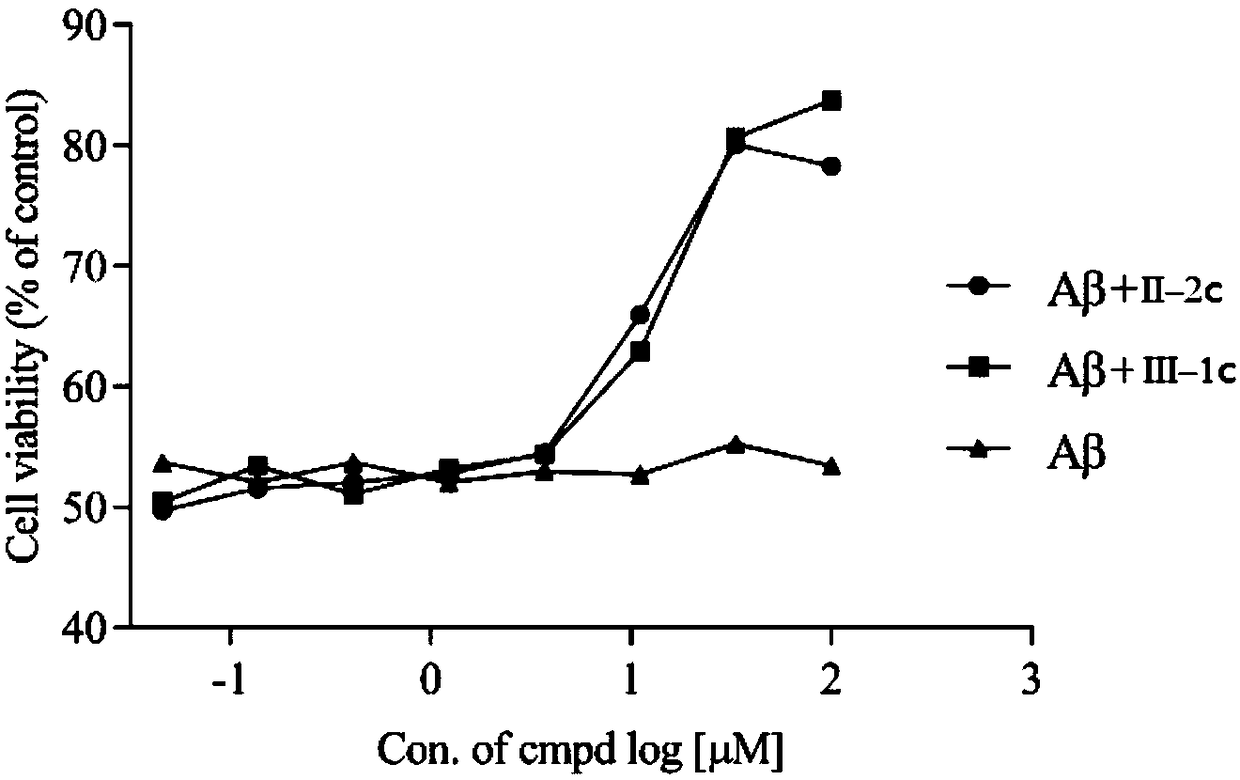
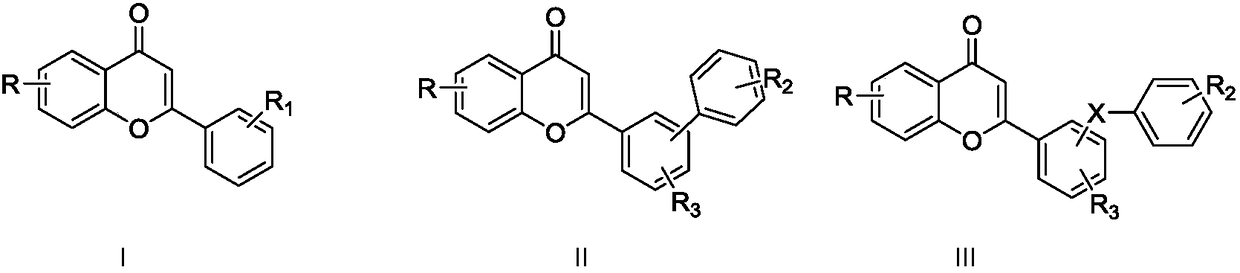
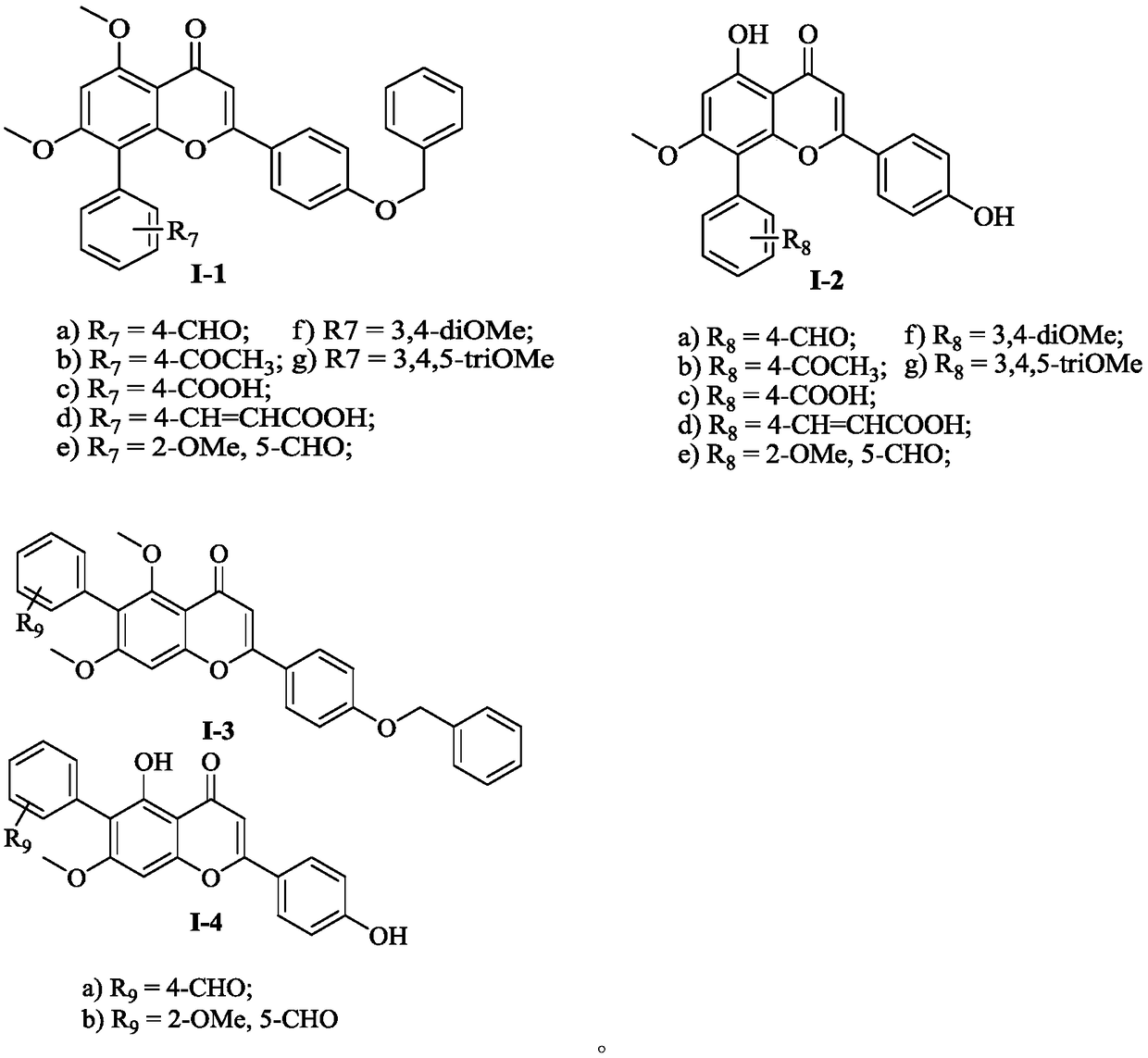
Flavone derivative and application thereof
A technology of derivatives and flavones, applied in the field of multi-target, multi-action anti-Alzheimer's flavone derivatives and its preparation, can solve problems such as unsatisfactory effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Embodiment 1: the preparation of compound II-3c
[0034] Chemical reaction formula:
[0035]
[0036] Synthesis of intermediate e
[0037] Weigh anhydrous zinc chloride powder (10.0g, 73.3mmol) into anhydrous acetonitrile (30g, 0.73mol), heat to 60-70°C, stir vigorously until no zinc chloride particles, then add phloroglucinol ( 20g, 0.15mol), after stirring and dissolving, the reaction solution was cooled to 0-5°C. Anhydrous diethyl ether (100 mL) was added, and dry hydrogen chloride gas was passed through for 12 h until no solid was formed. The reaction solution was placed in a refrigerator at 4 °C overnight. Suction filtration, the filter cake was washed with anhydrous ether. The filter cake was transferred to a flask in situ, water (1 L) was added, and after boiling for 2 hours, it was filtered while it was still hot. After the filtrate was cooled overnight, crystals were precipitated, filtered, and dried to obtain the product with a yield of 75%. 1 HNMR (50...
Embodiment 2
[0052] Embodiment 2: the preparation of compound I-2c
[0053]
[0054] Synthesis of intermediate j
[0055]Take 2-hydroxy-4,6-dimethoxyacetophenone (19.6g, 0.1mol), iodine element (11.4g, 0.045mol), and ethanol (200mL) into the reaction flask in sequence, and stir thoroughly. Take another HIO 3 (2.6g, 0.015mol) was dissolved in water (5mL), added to the reaction solution, and stirred well at room temperature for about 2.5 hours. After TLC stirring, the raw material point disappeared. Stop stirring and filter with suction. After the filtrate was concentrated to 1 / 2 volume, it was cooled to stand still, then suction filtered, and the filter cakes were combined to obtain a white solid with a yield of 93%. MS(ESI):m / z[M+H] + 322.98.
[0056] Synthesis of intermediate k
[0057] Dissolve 2-hydroxyl-3-iodo-4,6-dimethoxyacetophenone (32.2g, 0.1mol) and 4-benzyloxybenzaldehyde (21.2g, 0.1mol) in ethanol (350mL) and stir After dissolution, 30% KOH aqueous solution (100 mL) ...
Embodiment 3
[0064] Embodiment 3: the preparation of compound I-4a
[0065]
[0066] Synthesis of intermediate n
[0067] Intermediate m (0.69g, 1.77mmol) was weighed and dissolved in chloroform (70ml). After complete dissolution, tetrabutylammonium tribromide (TBATB, 0.85g, 1.77mmol) was added, stirred at room temperature, and monitored by TLC. After the reaction, the reaction solution was transferred to a separatory funnel. Wash with water and saturated brine successively, dry over anhydrous sodium sulfate, concentrate the organic phase, and perform column chromatography to obtain a yellow solid with a yield of 56%. m.p.230-232℃. 1 H NMR (500MHz, DMSO-d 6 ):δ H =8.07-8.03(m,2H),7.51-7.46(m,4H),7.41(t,J=7.4Hz,4H),7.38-7.33(m,2H),7.22-7.18(m,3H),6.77 (s,1H),6.74(s,1H),5.22(s,2H),4.04(s,3H),3.94(s,3H).MS(ESI):m / z[M+H] + 467.05.
[0068] Synthesis of Intermediate I-3a
[0069] Take compound n (5mmol), 4-carboxyphenylboronic acid (0.9g, 6mmol), anhydrous cesium carbonate (3.9g, 10m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com