Electroluminescent materials based on carbazole five-membered heterocycle units as well as preparation method and application of electroluminescent materials
A technology of electroluminescent materials and five-membered heterocycles, applied in the directions of luminescent materials, chemical instruments and methods, circuits, etc., can solve the problems of high price of OLED products, low utilization rate of materials, expensive equipment and other problems, and achieve high efficiency. The effect of stabilizing light-emitting device performance, easy chemical modification, improving fluorescence quantum yield and hole transport ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0074] 1. Preparation of carbazolo five-membered heterocyclic unit
[0075] (1) Synthesis of monomer M1
[0076] Preparation of ethylfuran-3-carboxylate: In a 500mL three-necked flask, dissolve furan-3-carboxylic acid (11.2g, 0.1mol) in 200mL of methanol, and add 20mL of concentrated sulfuric acid dropwise to the reaction solution. After stirring for 12 hours, stop the reaction, quench the reaction with water, extract with dichloromethane and dry with anhydrous magnesium sulfate. After the solution is concentrated, a yellow liquid is obtained, which is purified by silica gel column chromatography. The mixed solvent of petroleum ether / dichloromethane (5 / 1, v / v) was the eluent, and the yield was 73%. 1 H NMR, 13 C NMR, MS and elemental analysis results show that the compound obtained is the target product, and the chemical reaction equation of the preparation process is as follows:
[0077]
[0078] Preparation of ethyl 2-(tributyltin)furan-3-carboxylate: Under argon atmos...
Embodiment example 1
[0102] Implementation Case 1: Synthesis of Compound D1
[0103] Under argon atmosphere, in a 100mL three-necked flask, add monomer M1 (1.32g, 2.4mol), diphenylamine (0.85g, 5.0mmol), sodium tert-butyl alkoxide (1.84g, 19.2mmol), palladium acetate ( 27mg, 0.12mmol) and 50mL toluene. Heat and stir to 85° C., add 0.24 ml of 1 mol / L tri-tert-butylphosphine toluene solution, and react for 12 hours. After stopping the reaction, the solvent was concentrated, and the crude product was purified by column chromatography, using a mixed solvent of petroleum ether and dichloromethane (4 / 1, v / v) as the eluent, and finally an emerald green solid was obtained. 1 H NMR, 13 C NMR, MS and elemental analysis results show that the compound obtained is the target product D1, and the chemical reaction equation of the preparation process is as follows:
[0104]
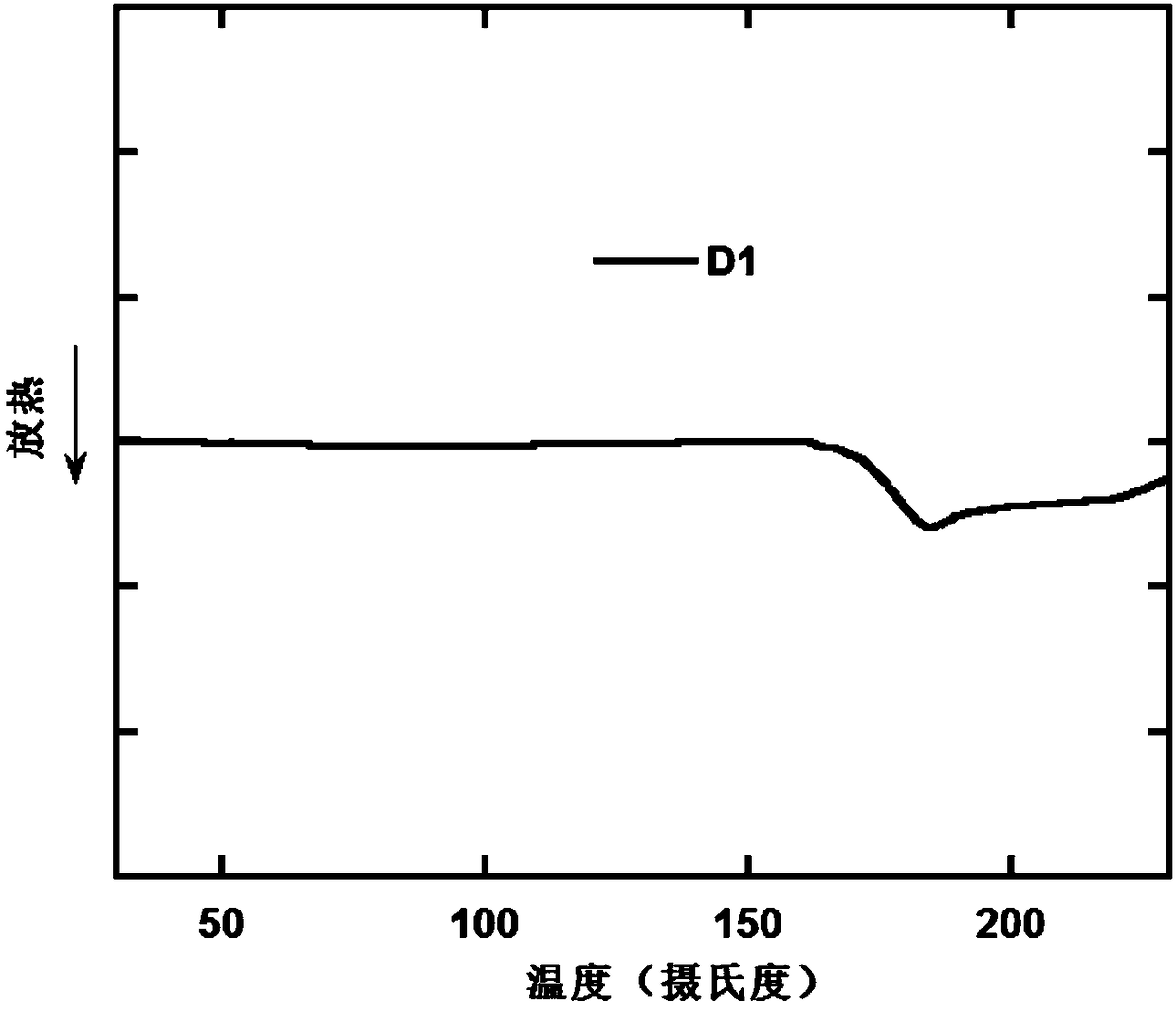
[0105] The differential scanning calorimetry (DSC) curve of compound D1 is as follows figure 1 shown. It can be seen from the figur...
Embodiment example 2
[0106] Implementation Case 2: Synthesis of Compound D2
[0107] The chemical reaction equation of the preparation process is as follows:
[0108]
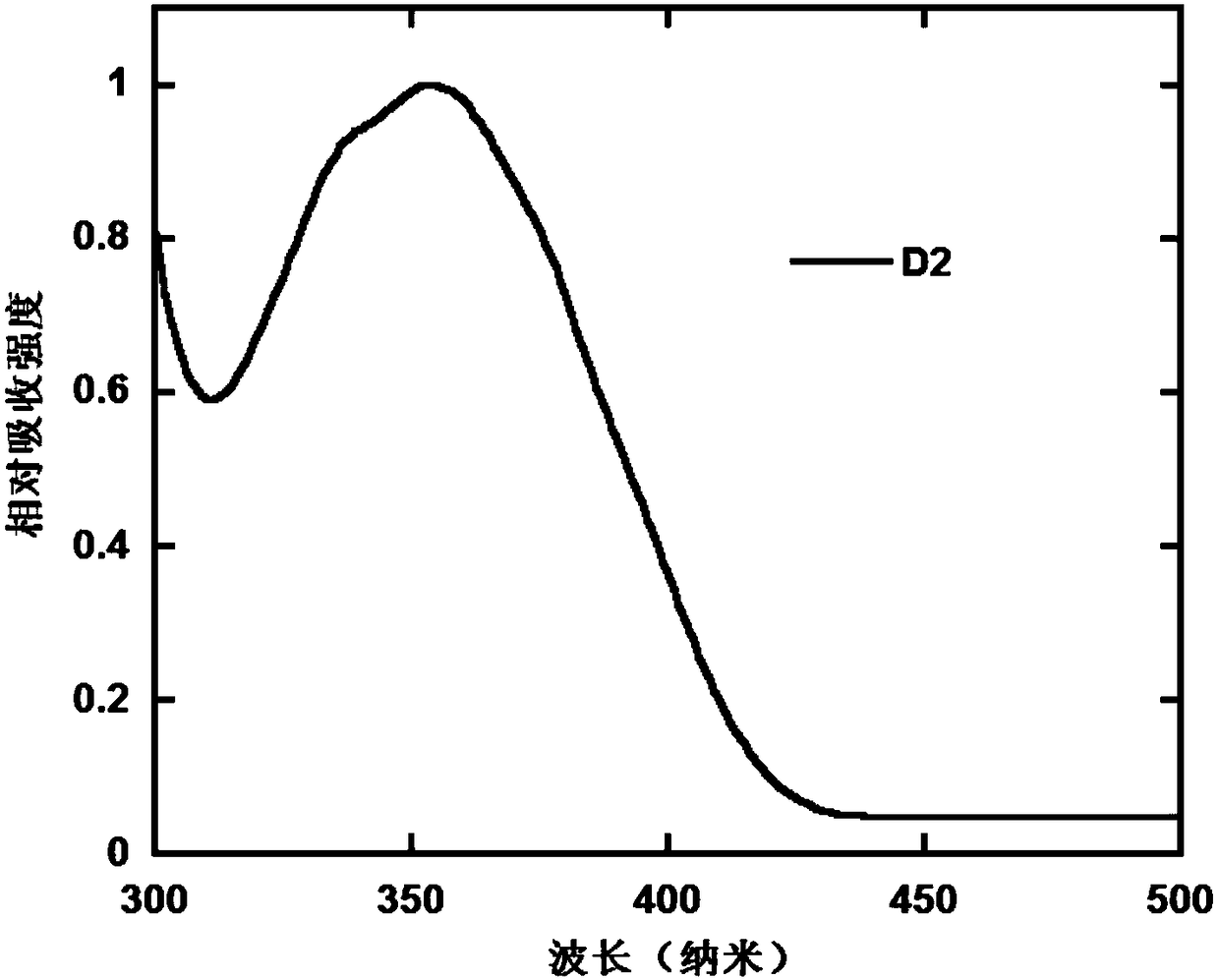
[0109] Under argon atmosphere, in a 100mL three-necked flask, add monomer M2 (1.32g, 2.4mol), diphenylamine (0.85g, 5.0mmol), sodium tert-butyl alkoxide (1.84g, 19.2mmol), palladium acetate (27mg , 0.12mmol) and 50mL toluene. Heat and stir to 85° C., add 0.24 ml of 1 mol / L tri-tert-butylphosphine toluene solution, and react for 12 hours. After stopping the reaction, the solvent was concentrated, and the crude product was purified by column chromatography, using a mixed solvent of petroleum ether and dichloromethane (4 / 1, v / v) as the eluent, to obtain an off-white solid. 1 H NMR, 13 The results of C NMR, MS and elemental analysis showed that the obtained compound was the target product D2. The ultraviolet-visible absorption spectrum of compound D2 in the thin film state is as follows: figure 2 shown by figure 2 It can be ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Glass transition temperature | aaaaa | aaaaa |
| Effective area | aaaaa | aaaaa |
| Maximum lumen efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com