Preparation method of nickel-ferrite nano material
A technology of nanomaterials and nickel ferrite, which is applied in nanotechnology, material resistance, chemical instruments and methods, etc., can solve problems such as high working temperature requirements, easy phase transition, poor selectivity, etc., and achieve high application value and preparation Simple method, good sensitivity and selectivity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Preparation of Ni-Fe-MOF Nanomaterials
[0020] Nickel nitrate hexahydrate (14.4 mg), ferric nitrate nonahydrate (29.2 mg), fumaric acid (123.7 mg), polyvinylpyrrolidone (75 mg) were dissolved in a mixed solution of N,N-dimethylformamide and ethanol ( 10mL, V / V=2:1). The above mixture was stirred at room temperature for 10 min, then the obtained solution was transferred to a 20 mL reactor and heated at 120 °C for 12 h, after centrifuging the obtained product, it was dried with DMF and ethanol in a vacuum oven at 60 °C for 8 h successively to obtain Precursor Ni-Fe-MOF nanomaterials.
Embodiment 2
[0022] Preparation of Ni-Fe-MOF Nanomaterials
[0023] Dissolve nickel nitrate hexahydrate (19.2mg), iron nitrate nonahydrate (36.4mg), terephthalic acid (182.6mg), polyvinylpyrrolidone (100mg) in a mixed solution of N,N-dimethylacetamide and ethanol (15mL, V / V=2:1). Stir the above mixture at room temperature for 10 min, then transfer the obtained solution to a 20 mL reaction kettle and heat at 140°C for 8 h, centrifuge the obtained product, centrifuge with DMF and ethanol successively, and dry in a vacuum oven at 80°C for 6 h , to obtain the precursor Ni-Fe-MOF nanomaterials.
[0024] The morphology of the obtained Ni-Fe-MOF nanomaterials was characterized by scanning electron microscopy. Such as figure 1 As shown, the obtained Ni-Fe-MOF nanomaterials have a biconical structure with a particle size of about 1.5 μm.
Embodiment 3
[0026] Preparation of NiFe 2 o 4 nanomaterials
[0027] The Ni-Fe-MOF nanomaterial (20 mg) in Example 1 or Example 2 was put into a crucible, placed in a muffle furnace, and calcined at a high temperature in an air atmosphere. The calcination temperature is 500°C, the heating rate is 1°C / min, and the calcination time is 4h.
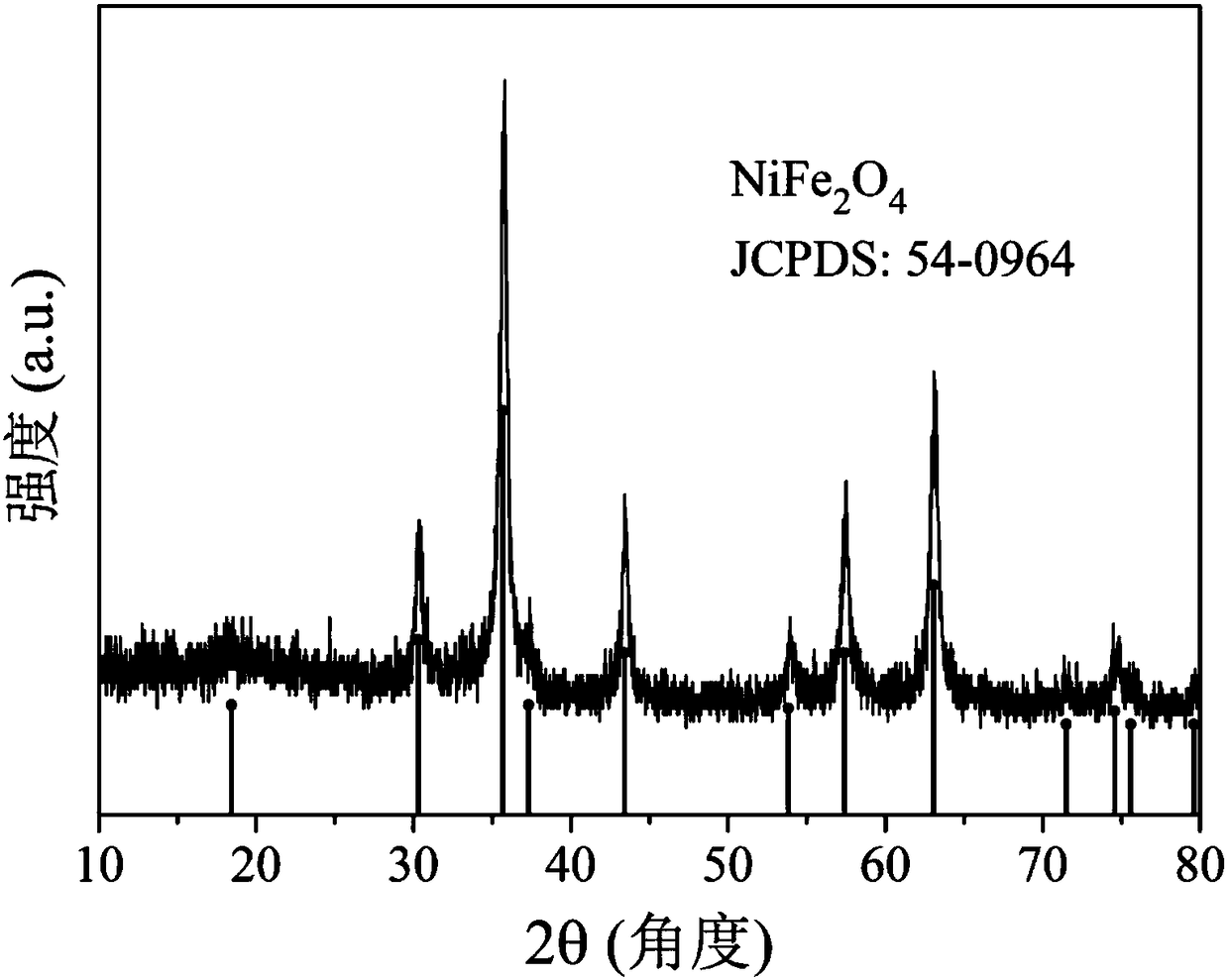
[0028] Its structure was analyzed by X-ray diffractometer, such as image 3 As shown, the nanomaterial is NiFe with spinel structure 2 o 4 nanomaterials.
[0029] Characterization of the obtained NiFe by scanning electron microscopy 2 o 4 Morphology of nanomaterials. Such as Figure 4 As shown, the resulting NiFe 2 o 4 The nanomaterial has a biconical structure, and the comparison before and after calcination shows that the material shrinks to a certain extent after calcination, and the aspect ratio increases.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com