PCR-specific primers, detection kits and detection methods for genes related to benign and malignant thyroid nodules
A technology for thyroid nodules and detection kits, applied in the field of molecular biology, can solve the problems of special customization of chips, poor repeatability, and complicated operations, so as to reduce the probability of being classified as malignant, reduce the operation of thyroid nodules, and improve the effect of sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Example 1 Multiplex PCR-specific primers, detection kit and detection method for genes related to benign and malignant thyroid nodules based on high-throughput sequencing technology
[0052] 1. Design of primers
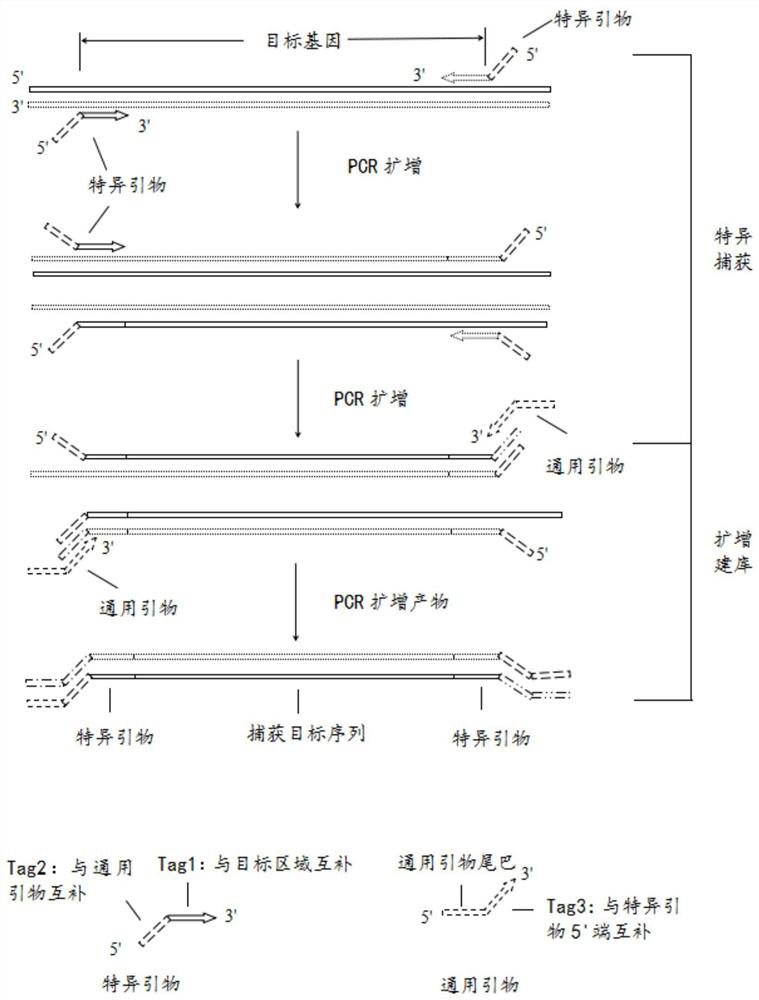
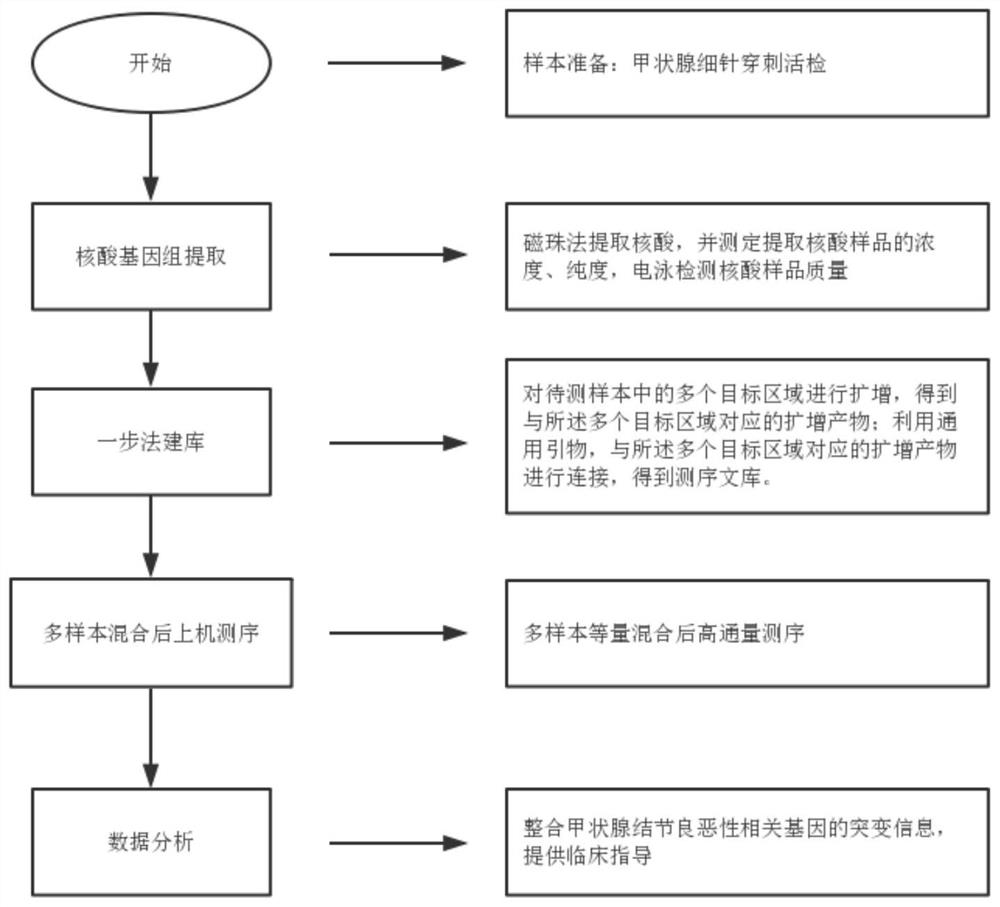
[0053] The gene sequences related to benign and malignant thyroid nodules involved in this example were selected from the UCSC (University of California Santa Cruz, University of California, Santa Cruz) database, and hotspot mutation primers were designed based on the relevant gene sequences, and the design range included benign and malignant thyroid nodules. Mutational hotspots in associated genes.
[0054] like figure 1 As shown, in this example, a total of 27 pairs of primers were designed for hotspot mutations of genes related to thyroid nodules. Wide coverage, stable structure, and multiple detection sites.
[0055] Specifically, 27 pairs of amplification primers are the amplification primer pairs of 24 genes in Table 1 as follows:
[0056] Table 1
...
Embodiment 2
[0081] Example 2 Primer Specificity Verification
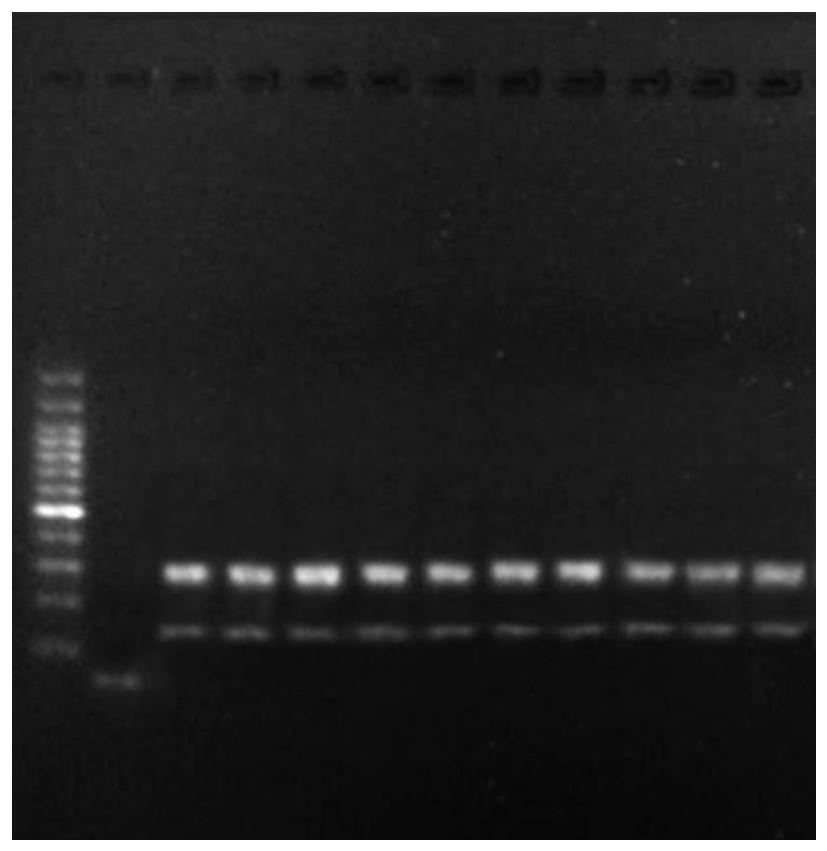
[0082] Nucleic acid was extracted from peripheral blood samples (number: 1-3), fine needle puncture samples (number: 4-6), and paraffin tissue samples (number: 7-9) using the method of step S11 in Example 1, and the concentration and purity were determined. After the determination, take qualified samples and use 10mM Tris to dilute each sample to 100ng / μL, and use 1% agarose gel electrophoresis to detect the quality of each sample (the qualification standard is the same as step S11 in Example 1), and enter the group after passing the test and perform mark. Using the method of step S12 in Example 1, the above-mentioned 9 cases of qualified samples were amplified, and the sample volume was 2 μL each. After the amplified product was purified, it was detected by 1% agarose gel electrophoresis (the qualification standard was the same as in Step S13 in Example 1. Step), 9 cases of samples were detected using specific primer amplifi...
Embodiment 3
[0084] Example 3 Primer Detection Sensitivity Verification
[0085] Using the method of step S11 in Example 1, sample nucleic acids were extracted from peripheral blood samples (number: 1), fine needle aspiration samples (number: 4), and paraffin tissue samples (number: 7) that passed the quality inspection to verify the sensitivity of primer detection. The initial concentration of each sample is 100ng / μL, and it is diluted according to the concentration gradient of 5 times, 10 times and 20 times. After dilution, the concentration of each sample is 20ng / μL, 10ng / μL and 5ng / μL respectively, and the sample name and Concentration marks. The method of step S12 in Example 1 was used to amplify the above-mentioned 9 cases of qualified diluted samples, and the sample volume was 2 μL each, and the 9 cases of samples were detected using specific primer amplification and detection methods for benign and malignant thyroid nodules related genes. The control test is the same as in Example...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com