A method for hydrogen production by phenol adsorption enhanced reforming
A technology for reforming hydrogen and phenol, which is applied in the energy field to achieve the effects of improved purity, good stability and high activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-5
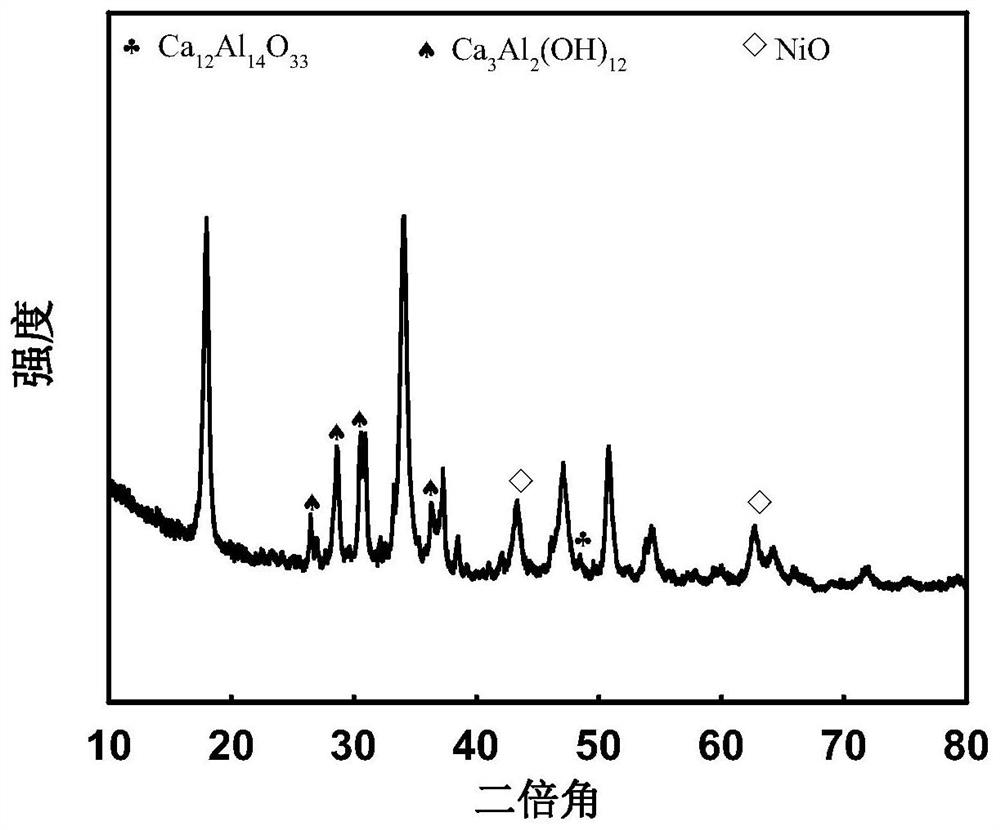
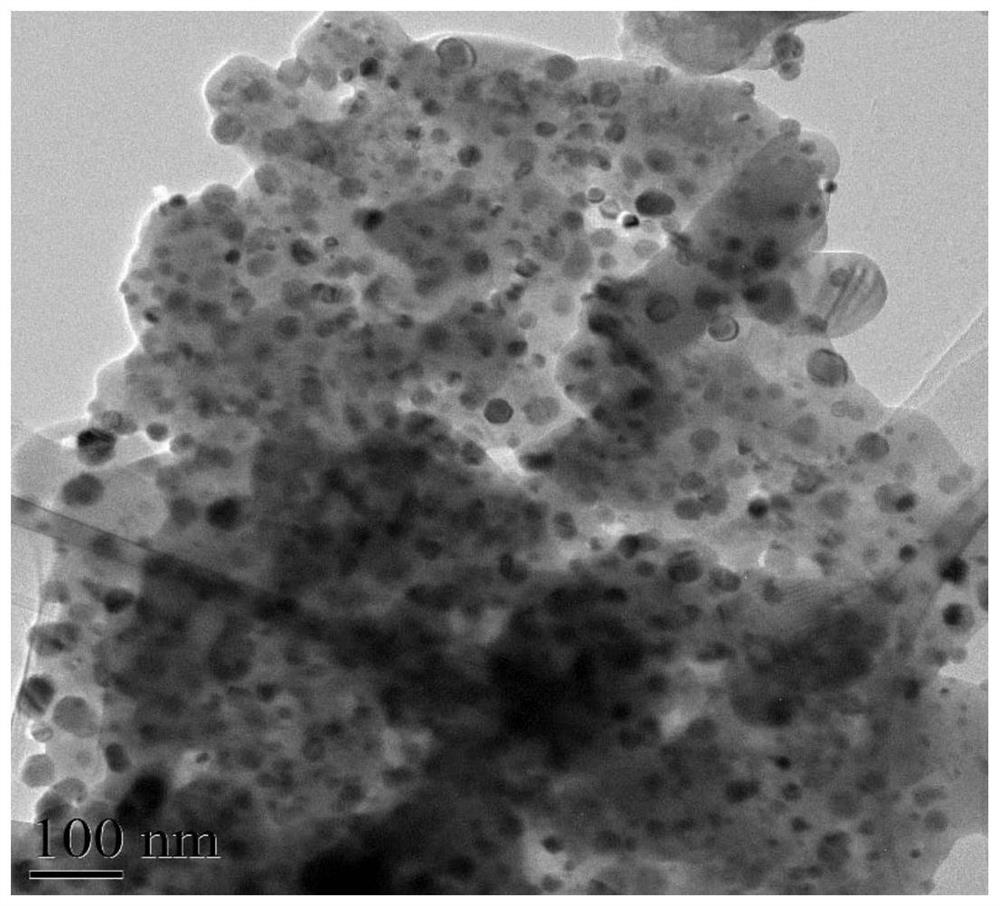
[0027] Weigh 0.0043mol of nickel nitrate, 0.064mol of the calcium salt in Table 1 and 0.023mol of the aluminum salt in Table 1, dissolve them in 50ml of water, dry at 120°C, and roast the obtained product in a muffle furnace at 800°C for 4h to obtain Ni -Ca-Al-O catalyst, Ni-Ca-Al-O catalyst packed in a fixed bed, argon as carrier gas, 1.296h -1 A phenol aqueous solution with a concentration of 0.0797 g / ml was introduced at a weight hourly space velocity of 0.0797 g / ml, and the reaction temperature was 550° C., and the purity of hydrogen in the product after 15 minutes of reaction was shown in Table 1 below. The X-ray diffraction figure, TEM figure of embodiment 1 gained Ni-Ca-Al-O catalyst are as follows figure 1 , figure 2 shown.
[0028] Table 1
[0029] Example 1 2 3 4 5 calcium salt calcium nitrate calcium nitrate calcium nitrate calcium phosphate calcium chloride Aluminum salt Aluminum nitrate Aluminum sulfate aluminum chlorid...
Embodiment 6-11
[0031] Take by weighing 0.0043mol nickel nitrate, 0.064mol calcium nitrate and 0.023mol aluminum nitrate, dissolve in 50ml water, dry at 120°C, and the product obtained is roasted under the conditions of Table 2 to obtain Ni-Ca-Al-O catalyst, in a fixed bed Fill Ni-Ca-Al-O catalyst in the medium, argon as carrier gas, take 1.296h -1 A phenol aqueous solution with a concentration of 0.0797 g / ml was introduced at a weight hourly space velocity of 0.0797 g / ml, the reaction temperature was 550° C., and the purity of hydrogen in the product after 15 minutes of reaction was shown in Table 2 below.
[0032] Table 2
[0033] Example 1 6 7 8 9 10 11 Calcination temperature (℃) 800 800 800 800 500 900 1000 Roasting time (h) 4 1 6 10 4 4 4 Hydrogen purity (%) 98.66 94.10 95.32 94.2 92.12 95.42 94.10
Embodiment 12-15
[0035] Weigh the calcium nitrate, aluminum nitrate and nickel nitrate shown in Table 3, dissolve them in 50ml of water, dry at 120°C, and roast the obtained product in a muffle furnace at 800°C for 4h to obtain Ni-Ca-Al-O catalyst. In the phenol adsorption enhanced steam reforming reaction, the Ni-Ca-Al-O catalyst was filled in the fixed-bed reactor, and argon was used as the carrier gas, with 1.296h -1 A phenol aqueous solution with a concentration of 0.0797 g / ml was introduced at a weight hourly space velocity of 0.0797 g / ml, and the reaction temperature was 550° C. After 15 minutes of reaction, the purity of hydrogen in the product and the conversion rate of phenol were shown in Table 3 below.
[0036] table 3
[0037] Example 12 1 13 14 15 Nickel nitrate (mol) - 0.0043 0.0086 0.0129 0.0172 Calcium nitrate (mol) 0.067 0.064 0.061 0.057 0.054 Aluminum nitrate (mol) 0.024 0.023 0.022 0.020 0.019 Hydrogen purity (%) 77.95 98...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com