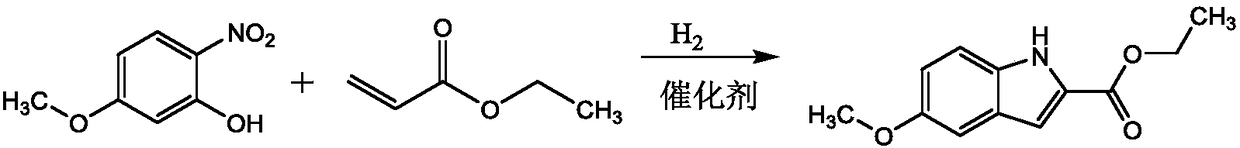
Preparation method for Arbidol intermediate
A technology of intermediates and preparation steps, which is applied in the field of preparation of Arbidol intermediates, can solve the problems of cumbersome operation, complex synthesis process, and low yield, and achieve the effects of simple process, high yield, and excellent product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
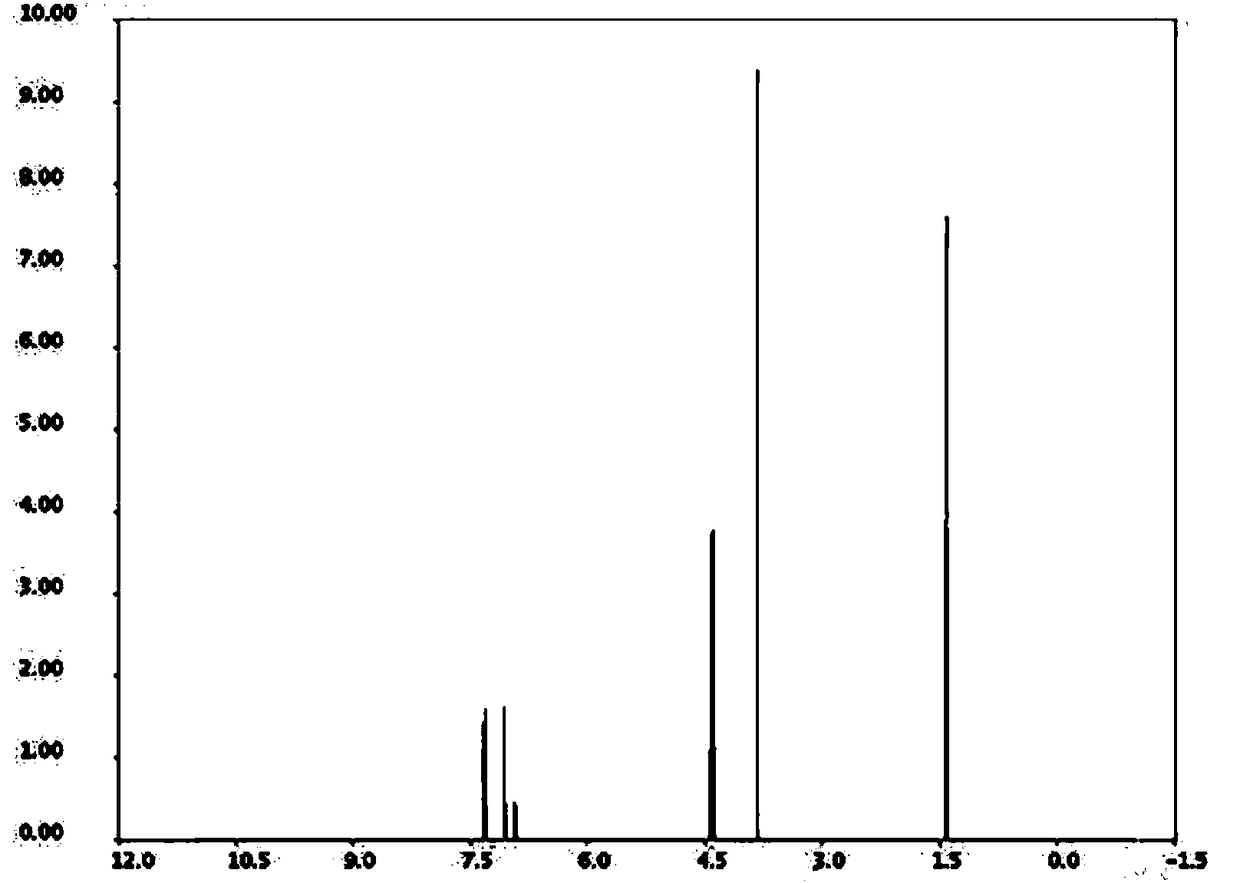
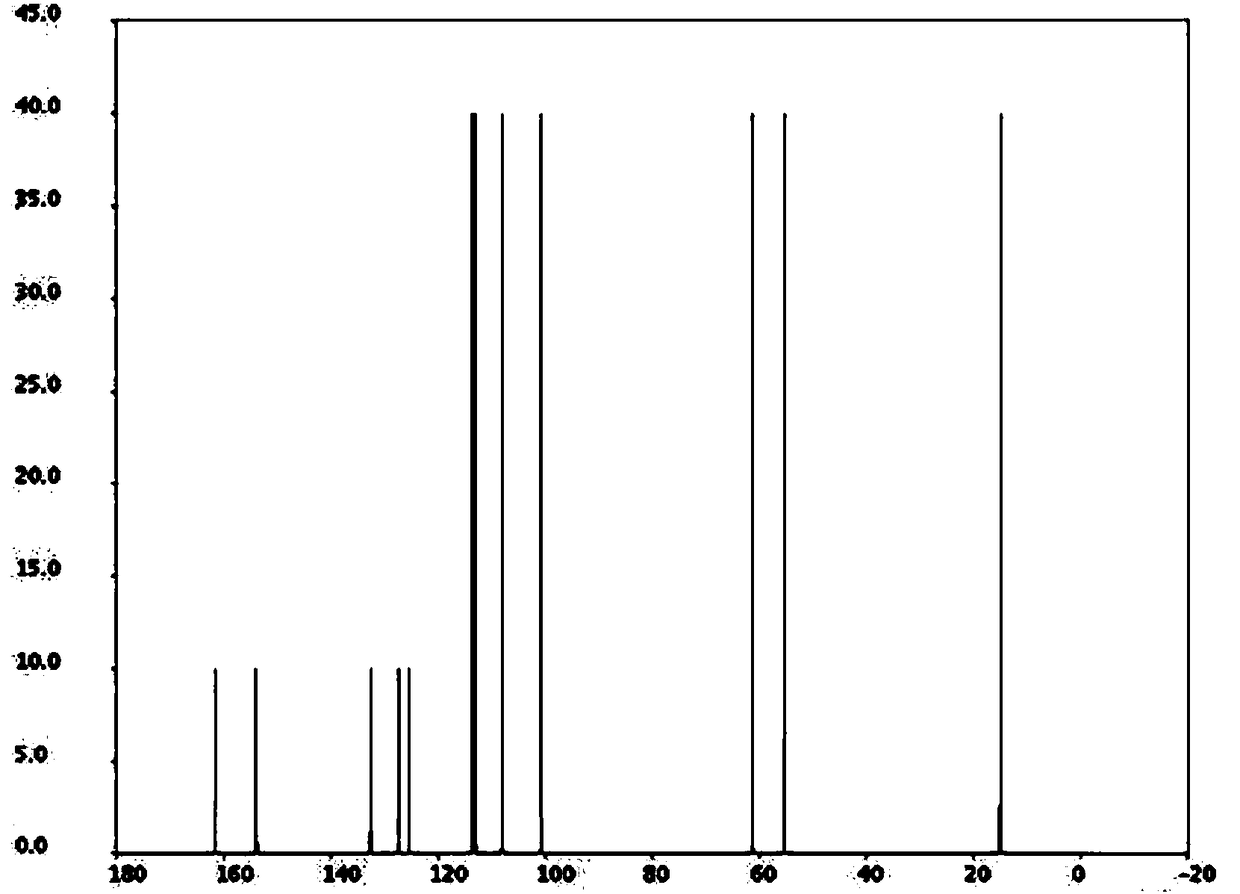
[0021] Weigh 5-methoxy-2-nitrophenol and ethyl acrylate at a molar ratio of 1:10, first put 5-methoxy-2-nitrophenol and ethyl acrylate into a three-necked flask, and then add The mass fraction is 30% acetic acid, the molar ratio of acetic acid and 5-methoxyl-2-nitrophenol is 4:1, the catalyst Pd / C of 5-methoxyl-2-nitrophenol mass 10%, successively Replace the gas in the three-necked flask with hydrogen and nitrogen for 2 times, exhaust the air in the bottle, and then pass hydrogen into the three-necked flask until the pressure in the three-necked flask is 0.3MPa, heat to 50°C, stir and react for 4 hours; recover after the reaction is completed Catalyst Pd / C, distill and recover excess ethyl acrylate, pour the reactants in the three-necked flask into cold water, filter with suction to obtain filter residue, wash and dry in an oven, and recrystallize after drying to obtain Arbidol Al intermediate, ethyl 5-methoxyindole-2-carboxylate.
example 2
[0023] Weigh 5-methoxy-2-nitrophenol and ethyl acrylate at a molar ratio of 1:13, first put 5-methoxy-2-nitrophenol and ethyl acrylate into a three-necked flask, and then add The mass fraction is 30% acetic acid, the molar ratio of acetic acid and 5-methoxyl-2-nitrophenol is 5:1, the catalyst Ru / C of 5-methoxyl-2-nitrophenol mass 13%, successively Replace the gas in the three-necked flask with hydrogen and nitrogen for 3 times, exhaust the air in the bottle, and then pass hydrogen into the three-necked flask until the pressure in the three-necked flask is 0.4MPa, heat to 55°C, stir and react for 5h; recover after the reaction is completed Catalyst Ru / C, distill and recover excess ethyl acrylate, pour the reactants in the three-necked flask into cold water, filter with suction to obtain filter residue, wash and dry in an oven, and recrystallize after drying to obtain Arbidol Al intermediate, ethyl 5-methoxyindole-2-carboxylate.
example 3
[0025] Weigh 5-methoxy-2-nitrophenol and ethyl acrylate at a molar ratio of 1:15, first put 5-methoxy-2-nitrophenol and ethyl acrylate into a three-necked flask, and then add Mass fraction is 30% acetic acid, the molar ratio of acetic acid and 5-methoxyl-2-nitrophenol is 6:1, the catalyst Raney nickel of 5-methoxyl-2-nitrophenol mass 15%, successively Replace the gas in the three-necked flask with hydrogen and nitrogen for 4 times, exhaust the air in the bottle, and then pass hydrogen into the three-necked flask until the pressure in the three-necked flask is 0.5MPa, heat to 60°C, stir and react for 6h; recover after the reaction is completed Catalyst Raney nickel, distill and recover excess ethyl acrylate, pour the reactants in the three-necked flask into cold water, filter with suction to obtain filter residue, wash and put it in an oven for drying, and recrystallize after drying to obtain Arbidol Al intermediate, ethyl 5-methoxyindole-2-carboxylate.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com