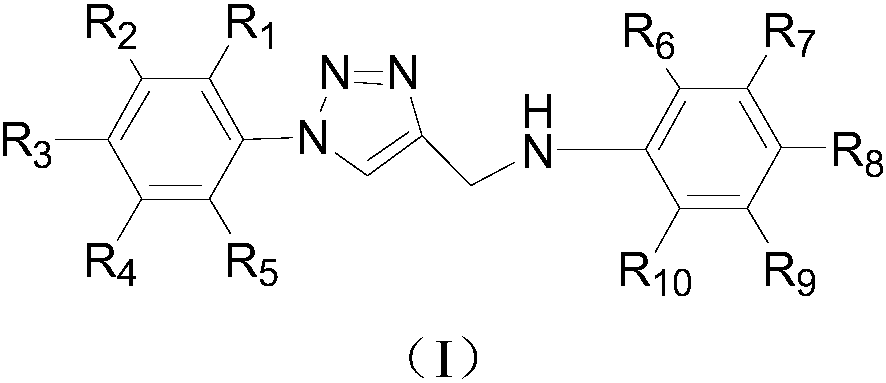
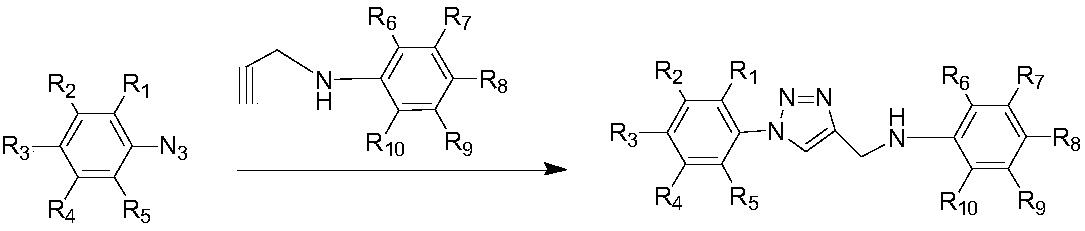
Deuterium labelled 1-substituted phenyl-4-substituted anilinomethyl-1,2,3-triazole derivatives as well as preparation method and application thereof
A technology of anilinomethyl and deuterium labeling, which is applied in organic chemical methods, chemical instruments and methods, drug combinations, etc., can solve the problems of limited application range, limited activity and no biological activity of anticancer drugs, and achieve good anticancer Biological activity, improvement of drug efficacy, and the effect of inhibiting cancer cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Embodiment 1: the synthesis of compound 1
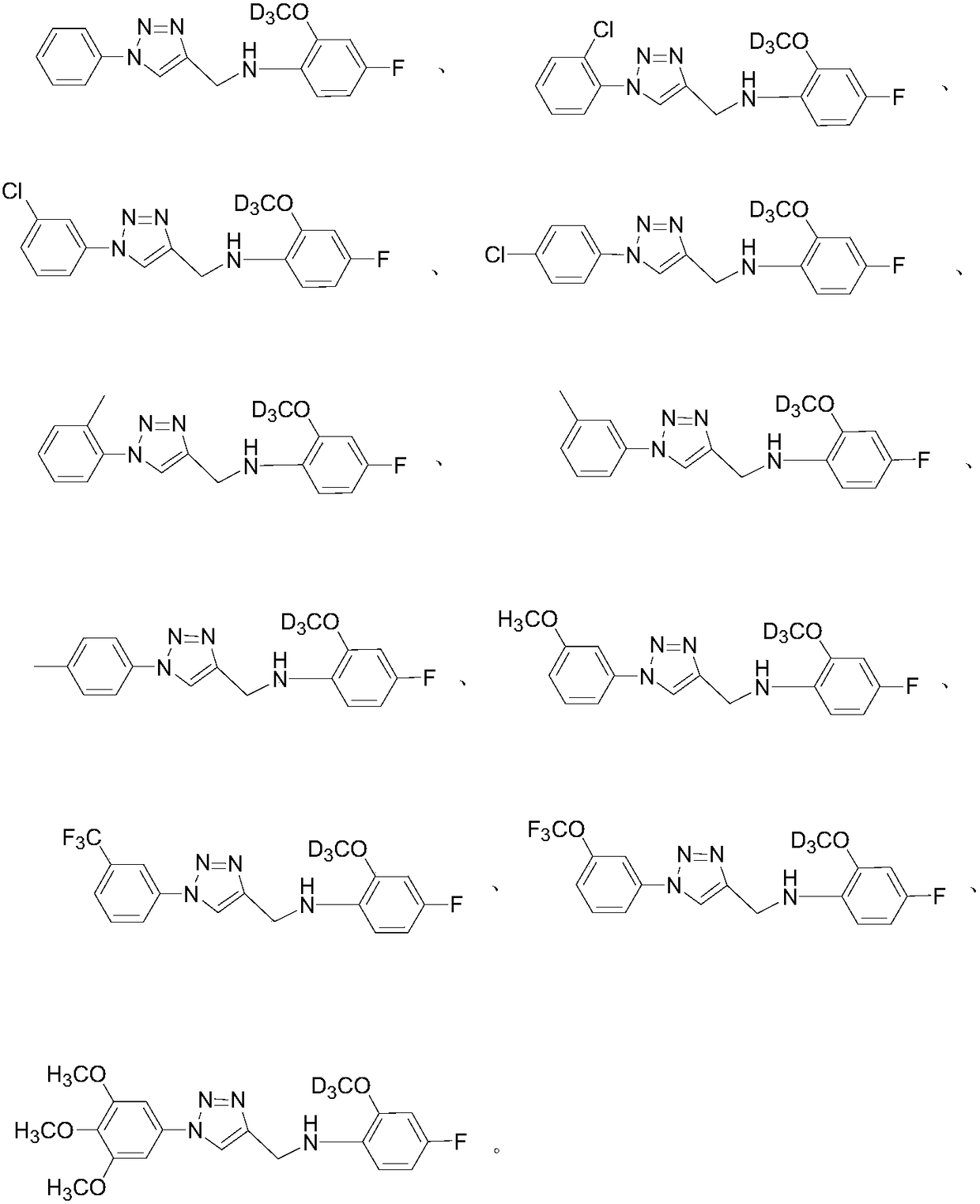
[0057] Add 0.26 g of benzene azido and 4-fluoro-2-methoxy-D in sequence 3 -N-(prop-2-yn-1-yl)aniline 0.20g, heavy water / isobutanol=5ml:5ml, sodium ascorbate 0.07g, copper sulfate pentahydrate 0.03g, react at room temperature, protect with nitrogen, after reacting for 0.5h (TLC tracking reaction), after the reaction was completed, an appropriate amount of ethyl acetate was added to extract three times, dried over anhydrous sodium sulfate, concentrated by rotary evaporation in vacuo, and column chromatographed to obtain 0.39 g of a brown-yellow product with a yield of 81%.
Embodiment 2
[0058] Embodiment 2: the synthesis of compound 2
[0059] Referring to Example 1, 1-azido-2-chlorobenzene and 4-fluoro-2-methoxy-D 3 -N-(prop-2-yn-1-yl)aniline was reacted to obtain compound 2 in a brownish yellow color with a yield of 68%.
Embodiment 3
[0060] Embodiment 3: the synthesis of compound 3
[0061] Referring to Example 1, 1-azido-3-chlorobenzene and 4-fluoro-2-methoxy-D 3 -N-(prop-2-yn-1-yl)aniline was reacted to obtain compound 3 in a brownish yellow color with a yield of 76%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com