Non-toxic cross-linked sodium hyaluronate gel articular cavity injection and preparation method thereof
A technology of sodium hyaluronate and articular cavity, which is applied in the direction of pharmaceutical formula, aerosol delivery, medical preparations of non-active ingredients, etc. It can solve problems such as toxicity, reduce toxicity, reduce the probability of adverse reactions, and have a stable structure Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
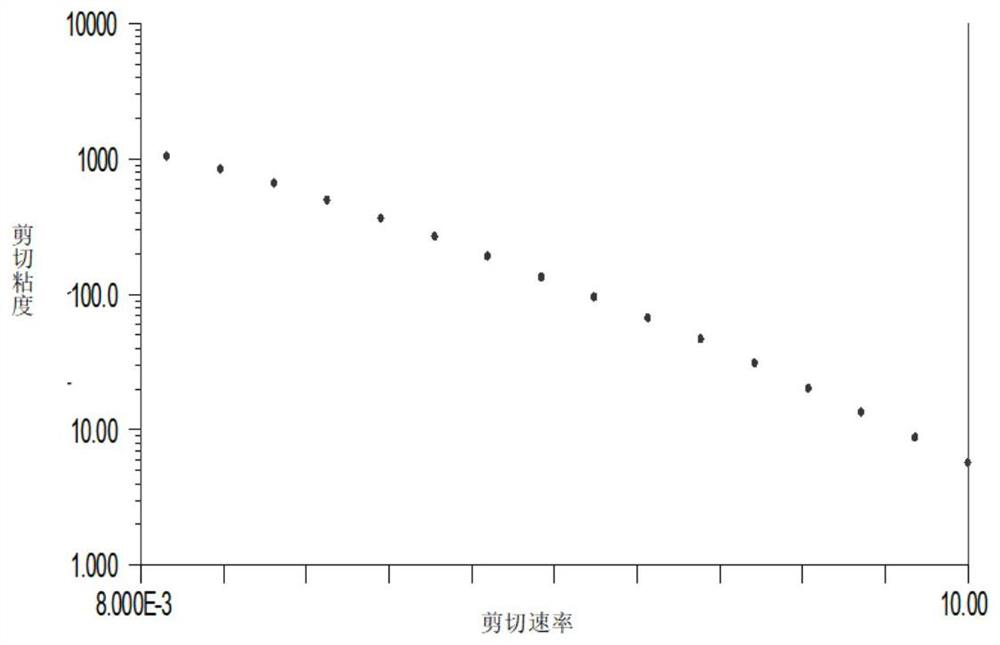
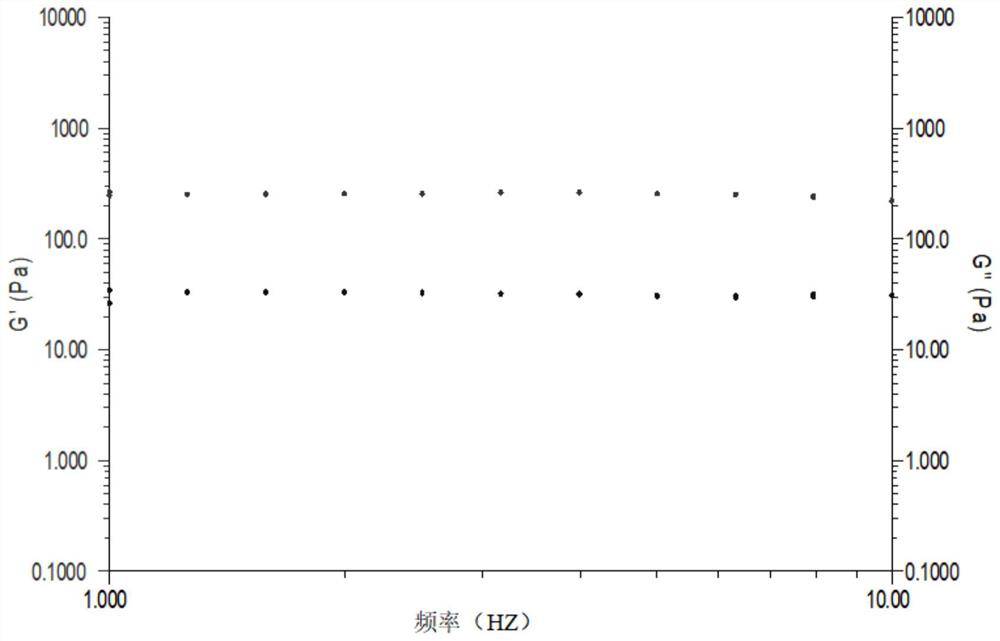
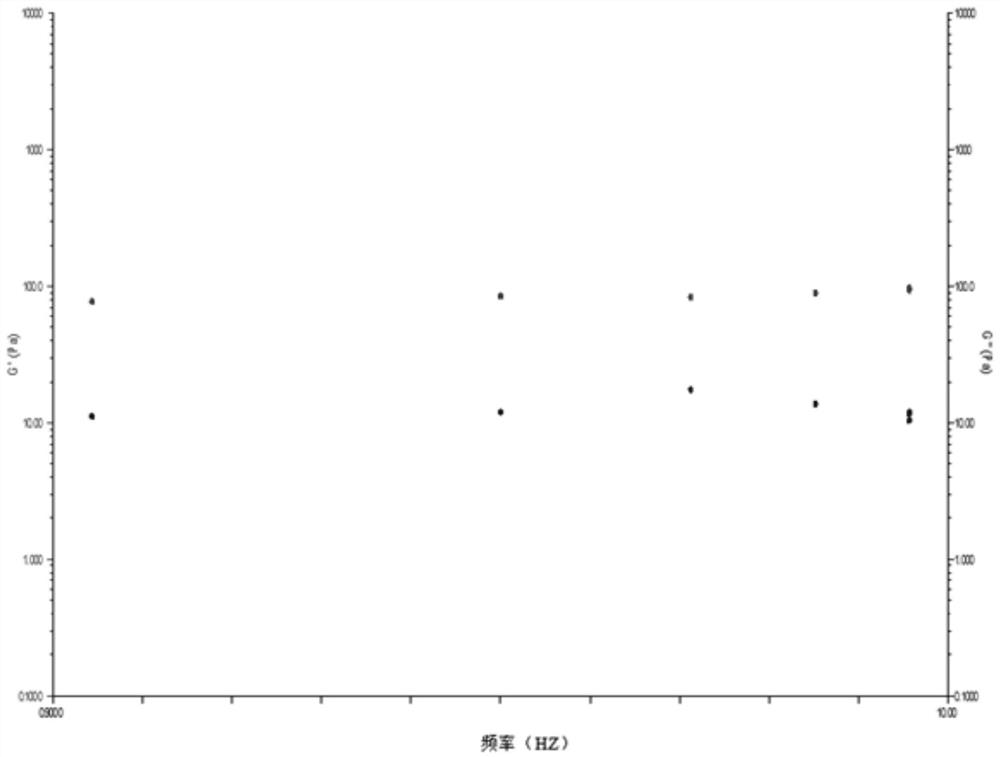
[0040] This example describes the preparation process of the cross-linking reaction between sodium hyaluronate and L-lysine, and detects the shear viscosity of the product.
[0041]Add 100 mL of phosphate buffer solution and 2 g of sodium hyaluronate raw material into a 500 mL round bottom flask, stir overnight at room temperature to dissolve. Add 1g of L-lysine into the reaction system, stir at 2-8°C for 2h, add 1g of DMTMM to catalyze the cross-linking reaction, and stir at 2-8°C for 24h to carry out the cross-linking reaction. Add ethanol to the obtained reaction product to precipitate and collect, and use ethanol to wash the obtained precipitate 3 times for 10 minutes each time, until the precipitate completely loses water, put the precipitate in a vacuum dryer and dry it for 24 hours to obtain a slightly cross-linked glass Sodium acid gel dry powder. Use phosphate buffer solution to redissolve the dry powder, add the dry powder to the phosphate buffer solution and dissol...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| cross-linking degree | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com