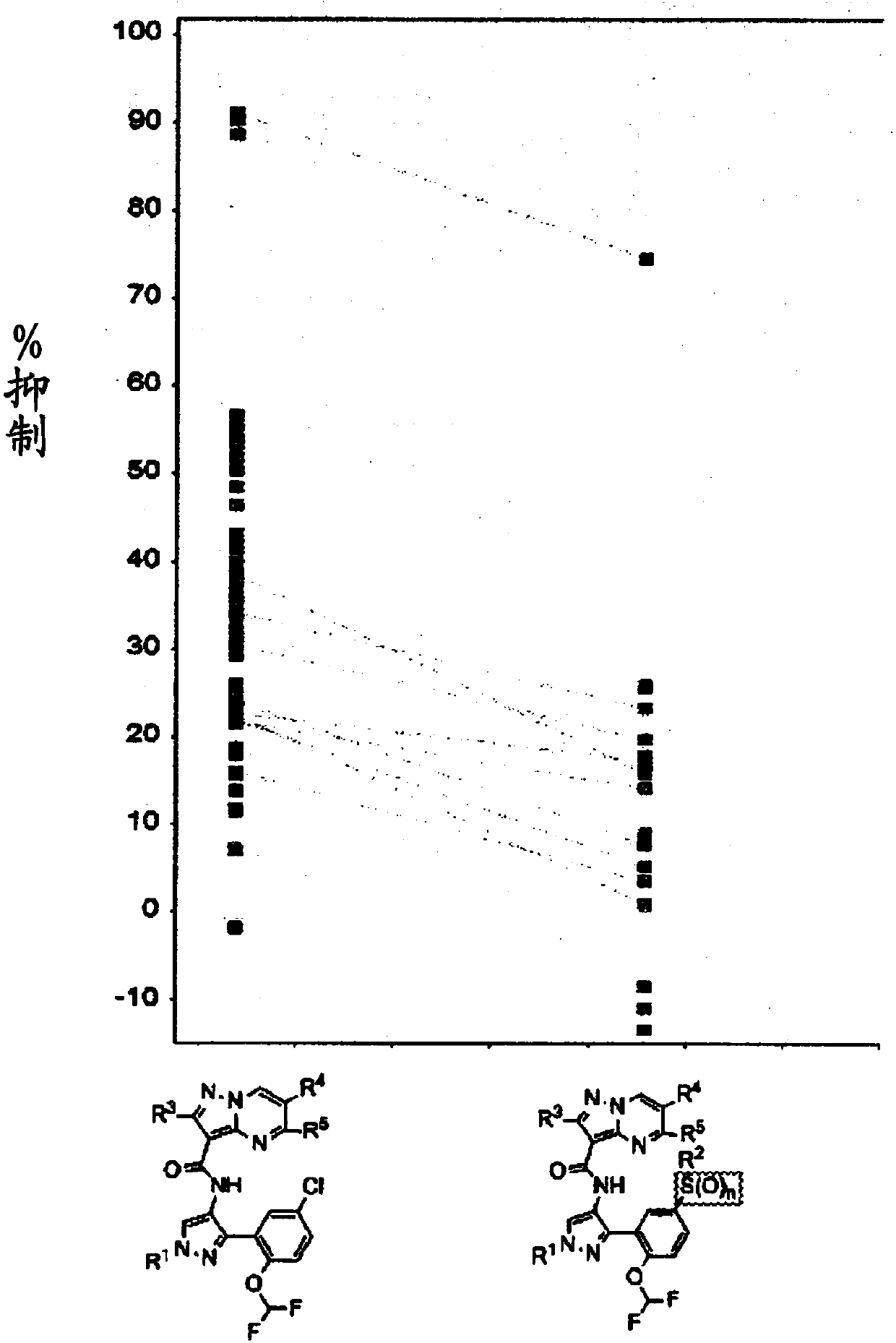
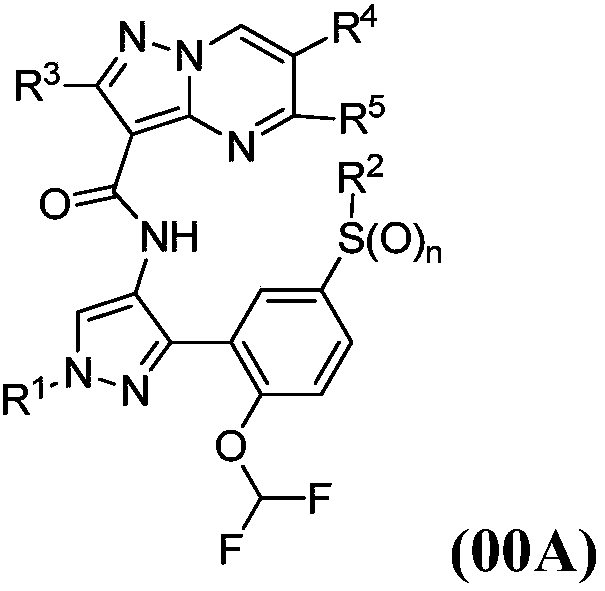
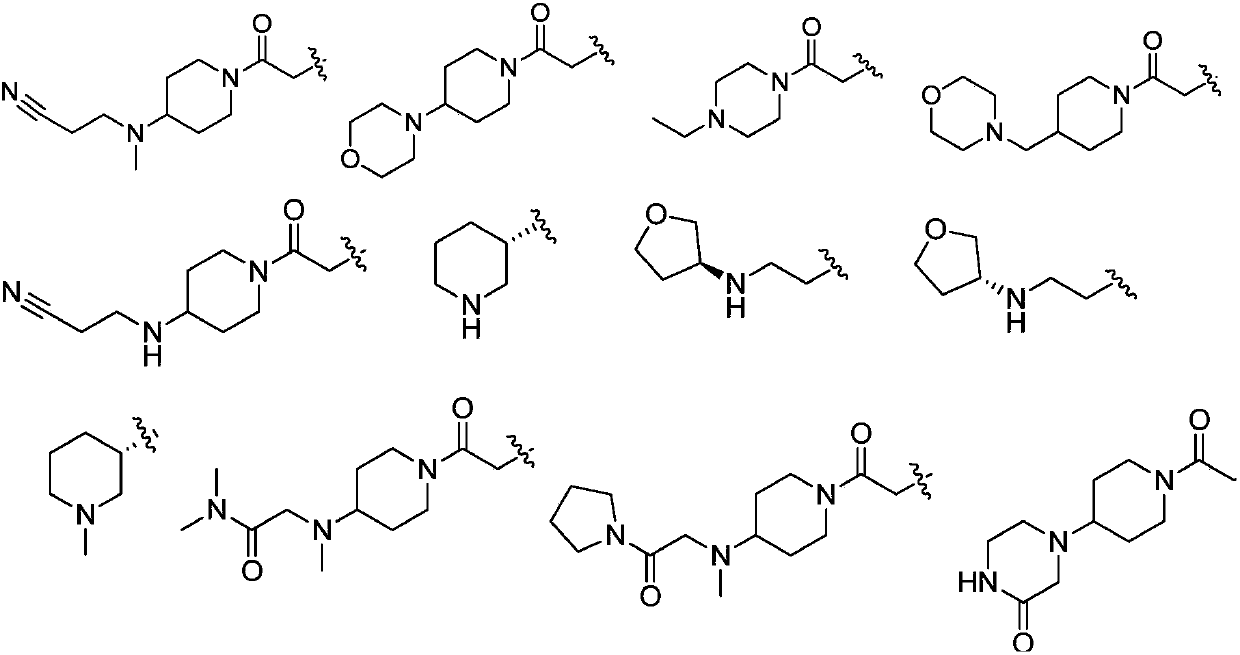
Janus kinases inhibitors, compositions thereof and use thereof
A compound, alkyl technology, used in drug combinations, anti-inflammatory agents, non-central analgesics, etc., can solve problems such as signal transduction inactivation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0455]
[0456] N-(3-(2-(Difluoromethoxy)-5-(methylthio)phenyl)-1H-pyrazol-4-yl)pyrazolo[1,5-a]pyrimidine-3- Formamide
[0457] To a solution of 4-bromo-2-iodophenol (282 g, 943.447 mmol) in N,N-dimethylformamide (2000 mL) and water (500 mL) was added sodium 2-chloro-2,2-difluoroacetate ( 216g, 1.417mol), Cs 2 CO 3 (617g, 1.894mol). The resulting mixture was stirred overnight at 120°C, allowed to cool to room temperature, and poured into ice water (3000ml). The resulting solution was extracted with ethyl acetate (3 x 1500ml), and the organic layers were combined. The ethyl acetate extract was washed with brine (1000ml), dried over anhydrous sodium sulfate and concentrated in vacuo. The residue was purified by flash chromatography on silica gel, eluting with ethyl acetate / petroleum ether (1:10), to obtain 300 g (91%) of 4-bromo-1-(difluoromethoxy)-2-iodobenzene as Yellow oil. 1 H NMR (300MHz, CDCl 3 )δ: (ppm) 7.96 (dd, J = 5.7Hz, 2.4Hz, 1H), 7.45 (dd, J = 8.7Hz, 2.4H...
Embodiment 5
[0490]
[0491] N-(3-(2-(difluoromethoxy)-5-(methylthio)phenyl)-1-(2-(4-morpholinopiperidin-1-yl)-2-oxo Ethyl)-1H-pyrazol-4-yl)pyrazolo[1,5-a]pyrimidine-3-carboxamide
[0492] In nitrogen atmosphere, to 2-[3-[2-(difluoromethoxy)-5-(methylsulfanyl)phenyl]-4-[pyrazolo[1,5-a]pyrimidine- 3-amino]-1H-pyrazol-1-yl]acetic acid (700mg, 1.48mmol), N,N-dimethylformamide (20mL) solution was added EDC.HCl (564mg, 2.94mmol), HOBt ( 400 mg, 2.96 mmol), DIPEA (762 mg, 5.90 mmol) and 4-(piperidin-4-yl)morpholine (502 mg, 2.95 mmol). The resulting solution was stirred overnight at room temperature. Additional amounts of EDC.HCl (564 mg, 2.94 mmol), HOBt (400 mg, 2.96 mmol) and DIPEA (762 mg, 5.90 mmol) were added. The resulting solution was stirred overnight at room temperature and concentrated in vacuo. The residue was applied to a silica gel column and eluted with dichloromethane / methanol (20 / 1-5 / 1). The appropriate fractions were combined and concentrated in vacuo. The residue was ...
Embodiment 11
[0494]
[0495] (R)-N-(3-(2-(Difluoromethoxy)-5-(methylthio)phenyl)-1-(2-((tetrahydrofuran-3-yl)amino)ethyl)- 1H-pyrazol-4-yl)pyrazolo[1,5-a]pyrimidine-3-carboxamide
[0496] Into a 500-mL round bottom flask flushed with nitrogen and maintained inert environment was added N-[3-[2-(difluoromethoxy)-5-(methylsulfanyl)phenyl]-1H-pyrazole- 4-yl]pyrazolo[1,5-a]pyrimidine-3-carboxamide (5.00g, 12.0mmol), tetrahydrofuran (150ml), Cs 2 CO 3 (15.7g, 48.2mmol,), 1,2-dibromoethane (45.0g, 240mmol). The reaction mixture was stirred at 80 °C for 2 h, cooled to room temperature and concentrated in vacuo. The crude product was recrystallized in a 3 / 1 mixed solvent of hexane / ethyl acetate. The solid was collected by filtration. 5.80 g (92%) of N-[1-(2-bromoethyl)-3-[2-(difluoromethoxy)-5-(methylsulfanyl)phenyl]-1H-pyridine were obtained Azol-4-yl]pyrazolo[1,5-a]pyrimidine-3-carboxamide as light yellow solid. LCMS (Method A, ESI): [M+H] + =525.1,R T =1.48min.
[0497] Add CH to a ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com