Bromopyridine dithiophene purple-blue fluorescent material
A bromopyridine dithiophene, blue fluorescence technology, applied in the field of luminescent materials, can solve the problems of complicated synthesis steps, harsh preparation conditions, expensive preparation and the like, and achieve the effects of simple synthesis steps, high luminous efficiency, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
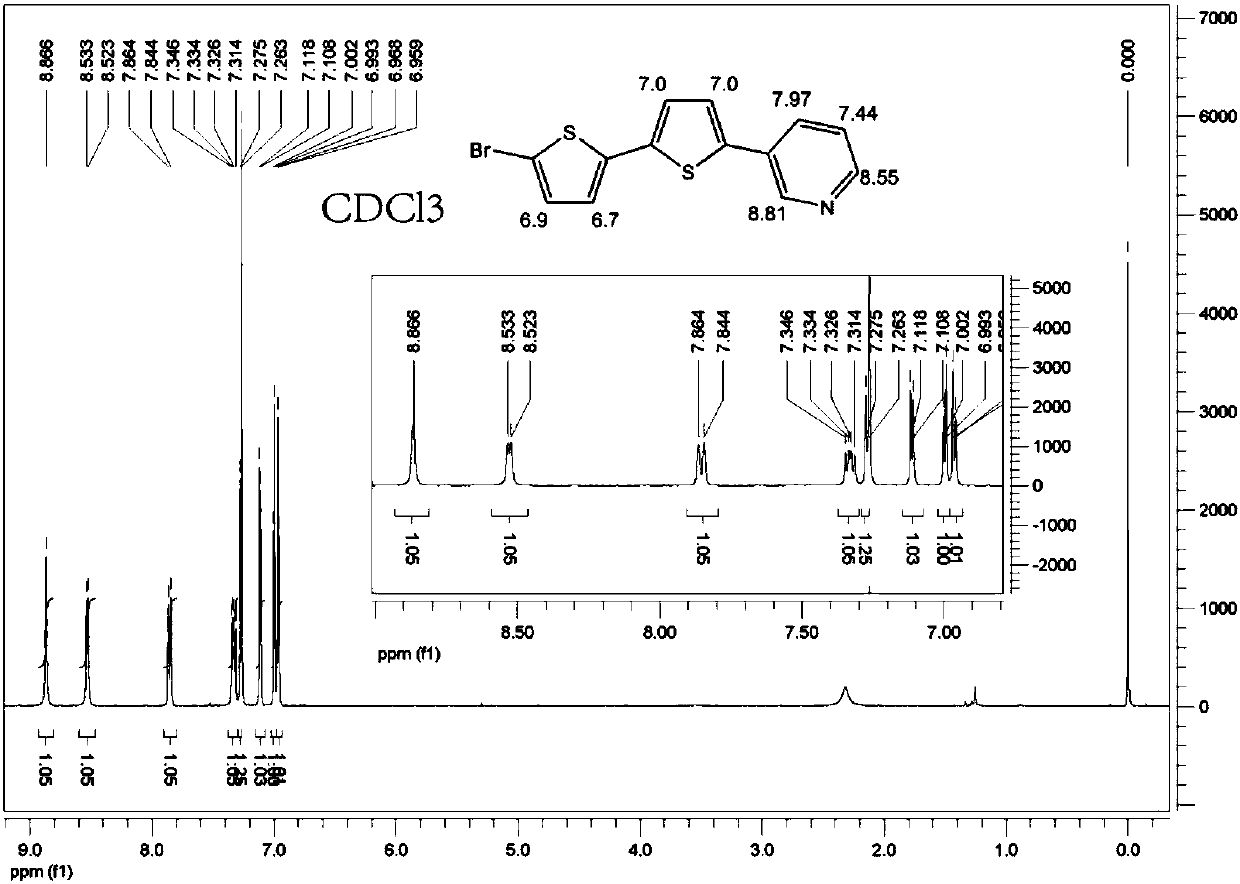
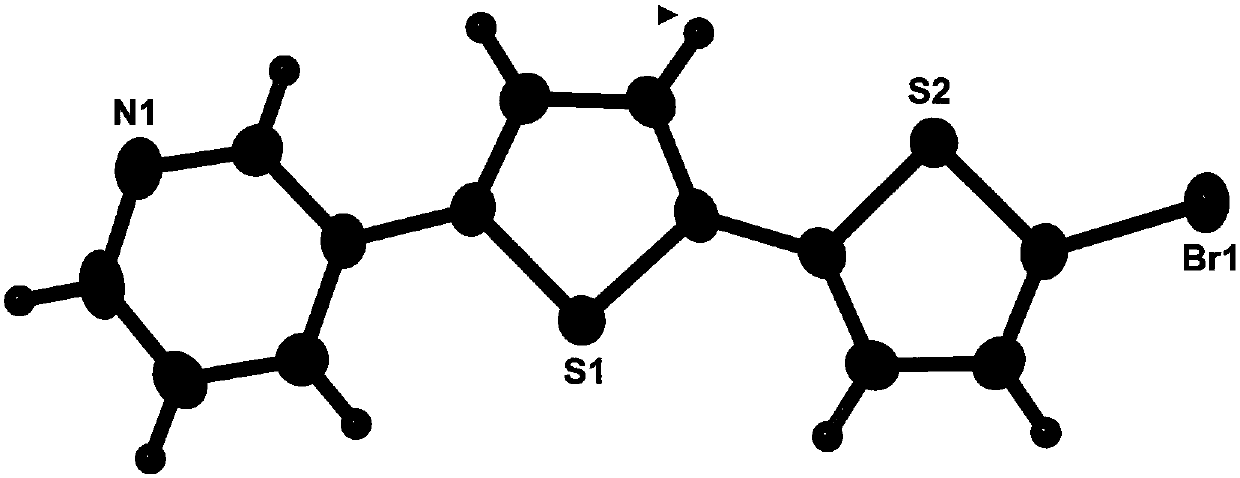
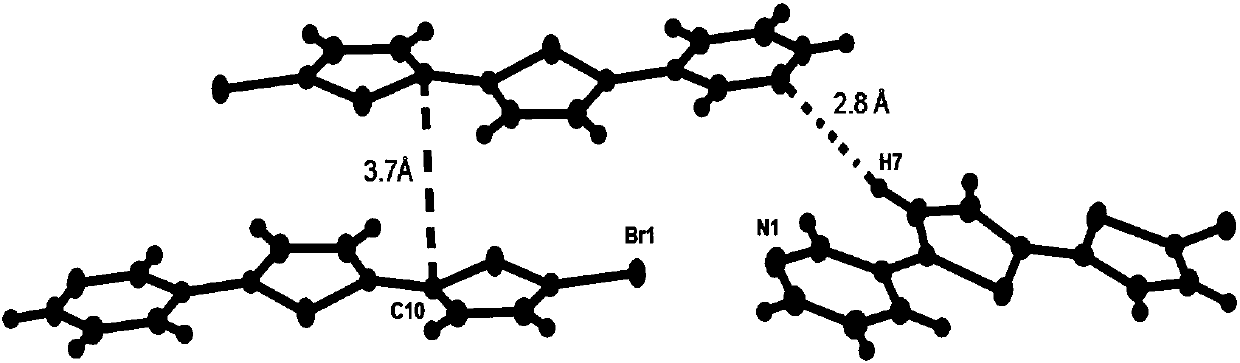
[0027] Take 5,5'-dibromo-2,2'-dithiophene (0.325g 1.0mmol), 3-pyridineboronic acid (0.246g 2.0mmol), tetrakis(triphenylphosphine)palladium (0.035g 0.03mmol) and carbonic acid Potassium (1.106g 8.0mmol) was mixed in a 100mL reaction device. After the reaction device was vacuumed, nitrogen gas was passed. Repeat the operation three times, and then add a mixed solution of 18 mL of toluene, 9 mL of ethanol, and 3 mL of water. The reaction was refluxed for 2 days. After the reaction was completed, the liquids were cooled and separated, extracted with dichloromethane, the organic phases were combined, washed with saturated brine and dried over magnesium sulfate to remove water. The solvent was removed by a rotary evaporator to obtain a solid powder, which was then separated through a column with an eluent of petroleum ether: ethyl acetate = 1:1 (volume ratio) to obtain a light yellow solid powder.
Embodiment 2
[0029] Take 5,5'-dibromo-2,2'-dithiophene (0.325g 1.0mmol), 3-pyridineboronic acid (0.271g 2.2mmol), tetrakis(triphenylphosphine) palladium (0.046g 0.04mmol) and carbonic acid Potassium (1.383g 10.0mmol) was mixed in a 100mL reaction device. The reaction device was evacuated and nitrogen was passed through. Repeat the operation three times, then add a mixed solution of 30 mL of toluene, 15 mL of ethanol, and 5 mL of water. The reaction was refluxed for 2 days. After the reaction was completed, the liquids were cooled and separated, extracted with dichloromethane, the organic phases were combined, washed with saturated brine and dried over magnesium sulfate to remove water. The solvent was removed by a rotary evaporator to obtain a solid powder, which was then separated through a column with an eluent of petroleum ether: ethyl acetate = 1:1 (volume ratio) to obtain a light yellow solid powder.
Embodiment 3
[0031] Take 5,5'-dibromo-2,2'-dithiophene (0.650g 2.0mmol), 3-pyridineboronic acid (0.738g 6.0mmol), tetrakis(triphenylphosphine)palladium (0.116g 0.1mmol) and carbonic acid Potassium (3.870g 28.0mmol) was mixed in a 100mL reaction device. The reaction device was evacuated and nitrogen gas was passed. Repeat the operation three times, and then add a mixture of toluene 36mL, ethanol 18mL, and water 6mL. Reflux reaction for 3 days. After the reaction was completed, the liquids were cooled and separated, extracted with dichloromethane, the organic phases were combined, washed with saturated brine and dried over magnesium sulfate to remove water. The solvent was removed by a rotary evaporator to obtain a solid powder, which was then separated through a column with an eluent of petroleum ether: ethyl acetate = 1:1 (volume ratio) to obtain a light yellow solid powder.
[0032] The substances participating in the reaction in the above examples are all chemically pure and above grade...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com