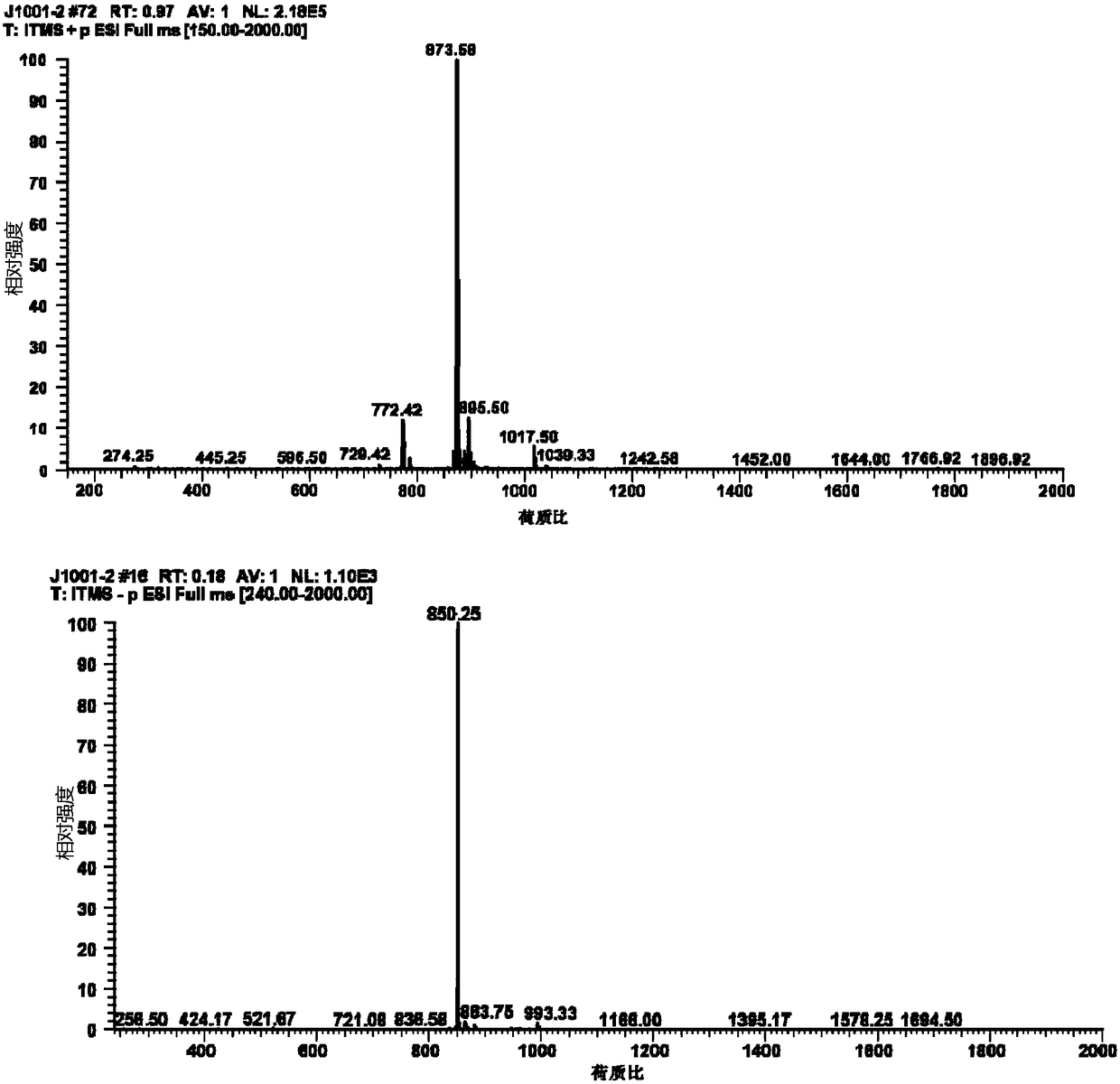
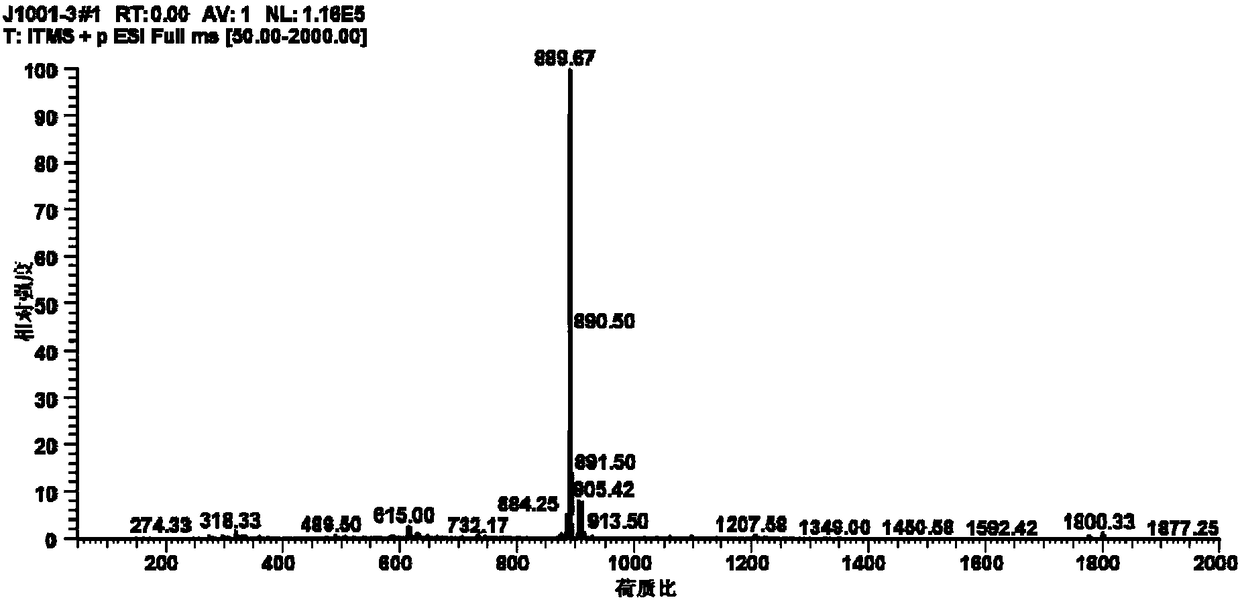
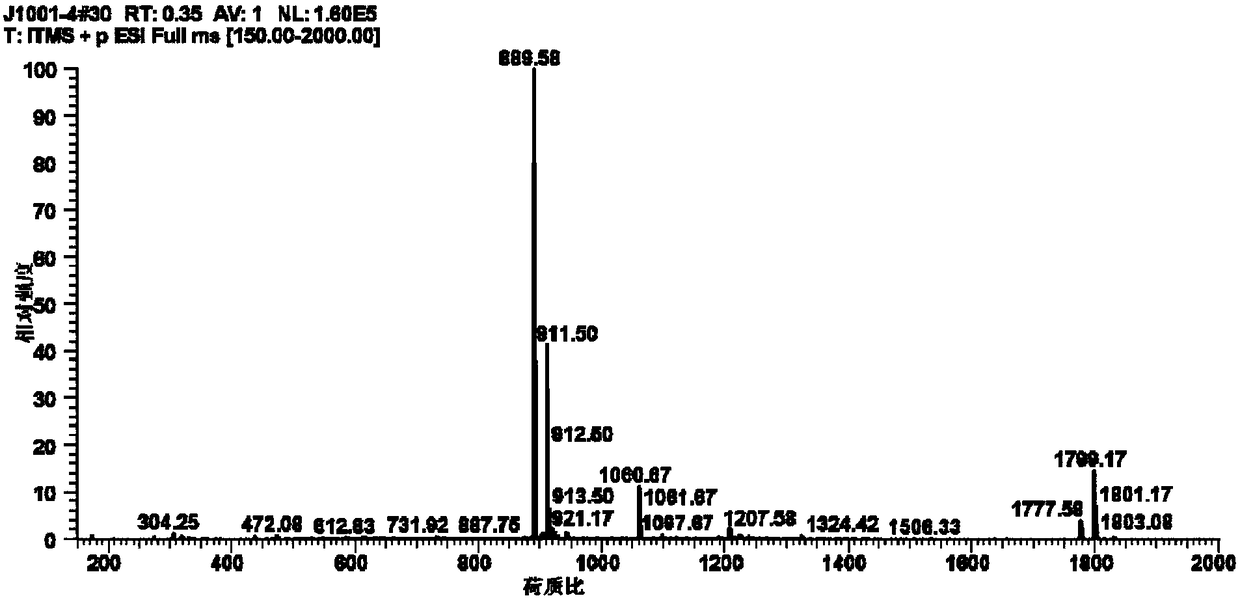
New mechanism research finding of glycosyl polyether compound as anti-cancer drug
A technology of compound and polyether, which is applied in the field of biomedicine, can solve the problem of the mechanism of anti-tumor activity to be studied, and achieve good anti-cancer effect and anti-proliferation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] The preparation method of embodiment 1 polyether compound
[0039] One Streptomyces nanchangensis subtype, two Streptomyces hygroscopicus subtypes and one Streptomyces endus subtype Streptomyces were inoculated on the SFM plate, and cultured for about three to four days. When colonies are formed in the SFM medium, pick a single colony and culture it in the seed medium for about three to four days, and inoculate it into the fermentation medium according to 1% amount (the composition of the fermentation medium is 30g / L of soluble starch, yellow Bean cake powder 10g / L, yeast extract 2.5g / L, CaCO 3 3g / L, pH 7.2). Culture conditions: 30 degrees, 220 rotation speed, culture for 7 to 8 days.
[0040] When the culture reaches the 7th or 8th day, collect the fermentation broth and centrifuge at a high speed to separate the supernatant from the mycelium, add an equal amount of acetone to the mycelium to destroy the bacteria, ultrasonicate for 20 minutes, spin evaporate acetone...
Embodiment 2
[0046] Example 2 Research on J1001-2 Sodium Salt by Proteomics and Phosphoproteomics
[0047] (1) Cell culture
[0048] MDA-MB-231 cells (triple-negative breast cancer cells) were cultured with DEME containing 10% fetal bovine serum, 80 μg / mL streptomycin, and 80 U / mL penicillin at 37°C and 5% CO 2 Routine cultivation in an incubator. When the cells are about 90% full, add the drug J1001-2 sodium salt. In the protein group experiment, 4 drug administration groups (administration concentration of 2 μM) were set up, which were 24h, 48h, 72h and blank group (no drug addition). In the phosphorylated protein group experiment, two administration groups (administration concentration of 3 μM) were set up for 6h, 12h and a blank group (no drug addition).
[0049] (2) Experimental methods of proteomics
[0050] 1) Proteome sample preparation
[0051] Collect the cells of the blank group and the administration group, transfer to a 1.5mL centrifuge tube, centrifuge at 2500rpm at 4°C ...
Embodiment 3
[0061] Example 3 Polyether compounds cause tumor cell reactive oxygen species (ROS) to increase
[0062] 1. Experimental method
[0063] MDA-MB-231 cells (triple-negative breast cancer cells) were cultured with DEME containing 10% fetal bovine serum, 80 μg / mL streptomycin, and 80 U / mL penicillin at 37°C and 5% CO 2 Routine cultivation in an incubator. When the cells are about 80% full, add the drug J1001-1 or J1001-2. The stock solution concentration of the drug is 50 mg / mL, and the drug is diluted to the set drug concentration with PBS. In the drug administration group, two drug concentrations (1 μM and 4 μM) are set, and the blank group (no drug added). Drug action time is 16 hours, repeated three times. Biyuntian active oxygen detection kit was used to detect according to the experimental steps provided by the kit, and the fluorescence intensity of the blank group and the drug-administered group was detected by flow cytometry to obtain Figure 7 The data.
[0064] 2. R...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com