Application of 2-phenyl-3-(p-aminophenyl)acrylonitrile as matrix in analyzing carbohydrates through MALDI-MS (Matrix Assisted Laser Desorption Ionization-Mass Spectrometry)
An aminophenyl, acrylonitrile technology, applied in the analysis of materials, material analysis by electromagnetic means, instruments, etc., can solve the problems of low ionization efficiency and inhibition, achieve simple synthesis, improve selectivity, and improve the degree of ionization Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
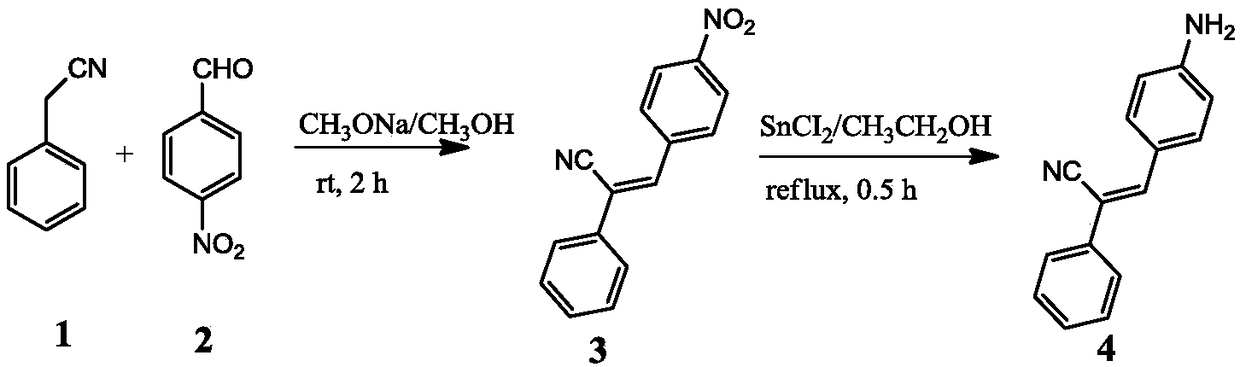
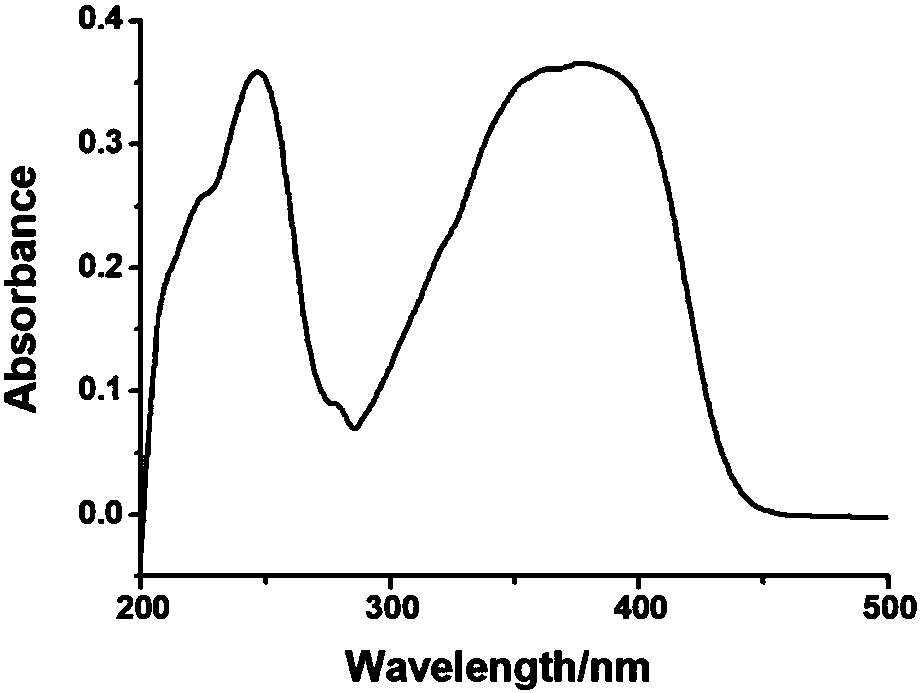
[0027] Example 1: Synthesis and analysis of PAPAN, the synthesis schematic diagram of PAPAN is as follows figure 1 As shown, the specific steps are as follows:
[0028] 1. First add 20mL of methanol and 0.115g, 0.005mol of metallic sodium into a round bottom flask, mix and stir until the metallic sodium is completely dissolved into sodium methoxide. Then add 1.17g, 0.01mol phenylacetonitrile, and 1.51g, 0.01mol p-nitrobenzaldehyde, stir the mixture at room temperature for 2h until the reaction of the raw materials is complete to form a yellow precipitate, and the filtered solid is washed three times with methanol and water to obtain 2.25g Bright yellow product.
[0029] 2. Add 2.5g, 0.01mol of the bright yellow product prepared in step 1, 40mL of ethanol and 11.3g, 0.05mol of stannous chloride dihydrate SnCl in a round bottom flask 2 2H 2 O, the mixture was refluxed for 0.5h. with saturated NaHCO 3 Neutralize to slightly alkaline, dilute with 50mL of water, then extract w...
Embodiment 2
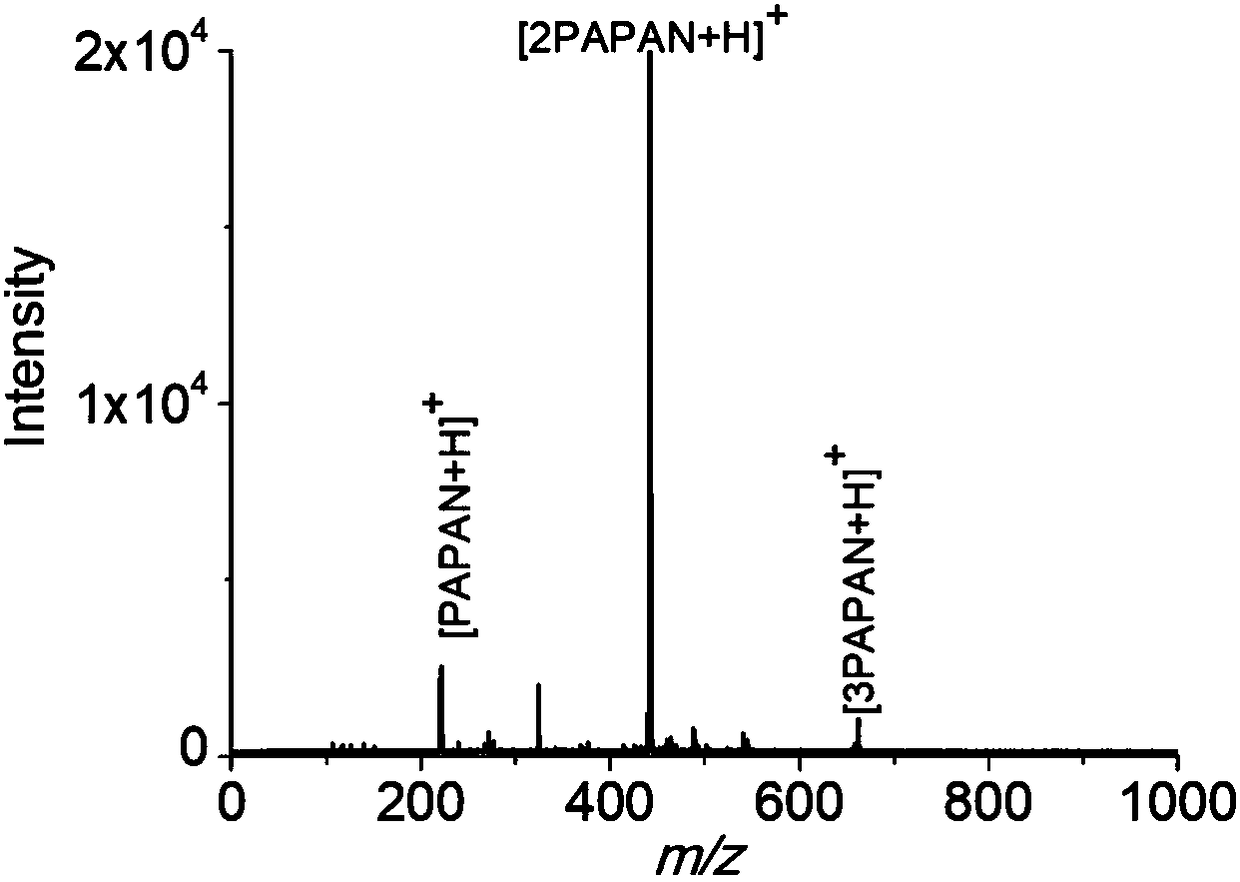
[0031] Example 2: PAPAN is used as a reactive matrix for the detection of maltohexaose by MALDI-MS.
[0032] 1. Prepare 10mM maltohexaose mother solution (aqueous solution), which can be stored in a refrigerator at 4°C.
[0033] 2. Configure 2.5g / L PAPAN solution in Example 1, the solvent used is methanol solution containing 5% acetic acid. It can be stored in a refrigerator at 4°C, protected from light.
[0034] 3. Take 99 μL of the PAPAN solution in step 2 into a 1.5 mL centrifuge tube, then add 1 μL of the maltohexaose mother liquor in step 1, mix well, and place it in a 65°C water bath for 1 hour.
[0035] 4. Take 0.5 μL of the reaction solution in step 3 and spot it on the anchorchip target plate, and immediately take 0.5 μL of pure water and spot it on the reaction solution, and let it dry naturally at room temperature.
[0036] 5. As a comparison, the mother liquor of maltohexaose in step 1 was diluted to 10 -4 M, take 1 μL maltohexaose solution and 1 μL common substra...
Embodiment 3
[0039] Example 3. Comparison of crystal morphology formed by two spotting methods when PAPAN is used as a matrix.
[0040] 1. Take 0.5 μL of the reaction solution in Example 2 and spot it on the anchorchip target plate, then immediately take 0.5 μL of pure water and spot it on the reaction solution, before the reaction solution dries, let it dry naturally at room temperature.
[0041] 2. Take 0.5 μL of the reaction solution in Example 2 and spot it on the anchorchip target plate, and let it dry naturally at room temperature.
[0042] 3. Send the target plate into MALDI-MS to observe its morphology.
[0043] Figure 5 The figure on the left is the MALDI-MS self-contained microscope morphology image obtained by applying the method of step 1 in Example 3, Figure 4 The right side is the morphological diagram obtained by spotting the method in step 2 of Example 3. It can be seen that the spotting method adopted in the present invention forms more uniform crystals than the common...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com