Cell culture medium and preparation method thereof
A culture medium and cell technology, applied in cell culture medium, cell culture active agents, biochemical equipment and methods, etc., can solve the problems of difficult separation, purification and detection of culture products, limited sources of animal serum, and restrictions on large-scale use, etc. Achieve the effect of improving cell proliferation, promoting wound healing, and good biocompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0025] Preparation of sericin solution: Sericin with a purity of more than 92% is dissolved in water or PBS buffer solution to obtain a sericin stock solution with a concentration greater than 100 mg / ml. The stock solution is used after being sterilized by conventional high temperature sterilization method or sterilized by 0.2 μm filter.
[0026] The phytohemagglutinin is selected from kidney bean phytohemagglutinin.
[0027] The preparation method of this culture medium comprises the following steps:
[0028] 4) Accurately weigh the dry powder of basic medium, chitosan quaternary ammonium salt, vitamin C, vitamin E, human serum albumin, and phytohemagglutinin of corresponding concentrations and dissolve them in an ultra-pure medium with a volume of 800ml and a temperature of 20-30°C. In water, stir until completely dissolved to obtain solution A;
[0029] 5) Add corresponding concentrations of sericin solution, epidermal growth factor, insulin-like growth factor, and L-glut...
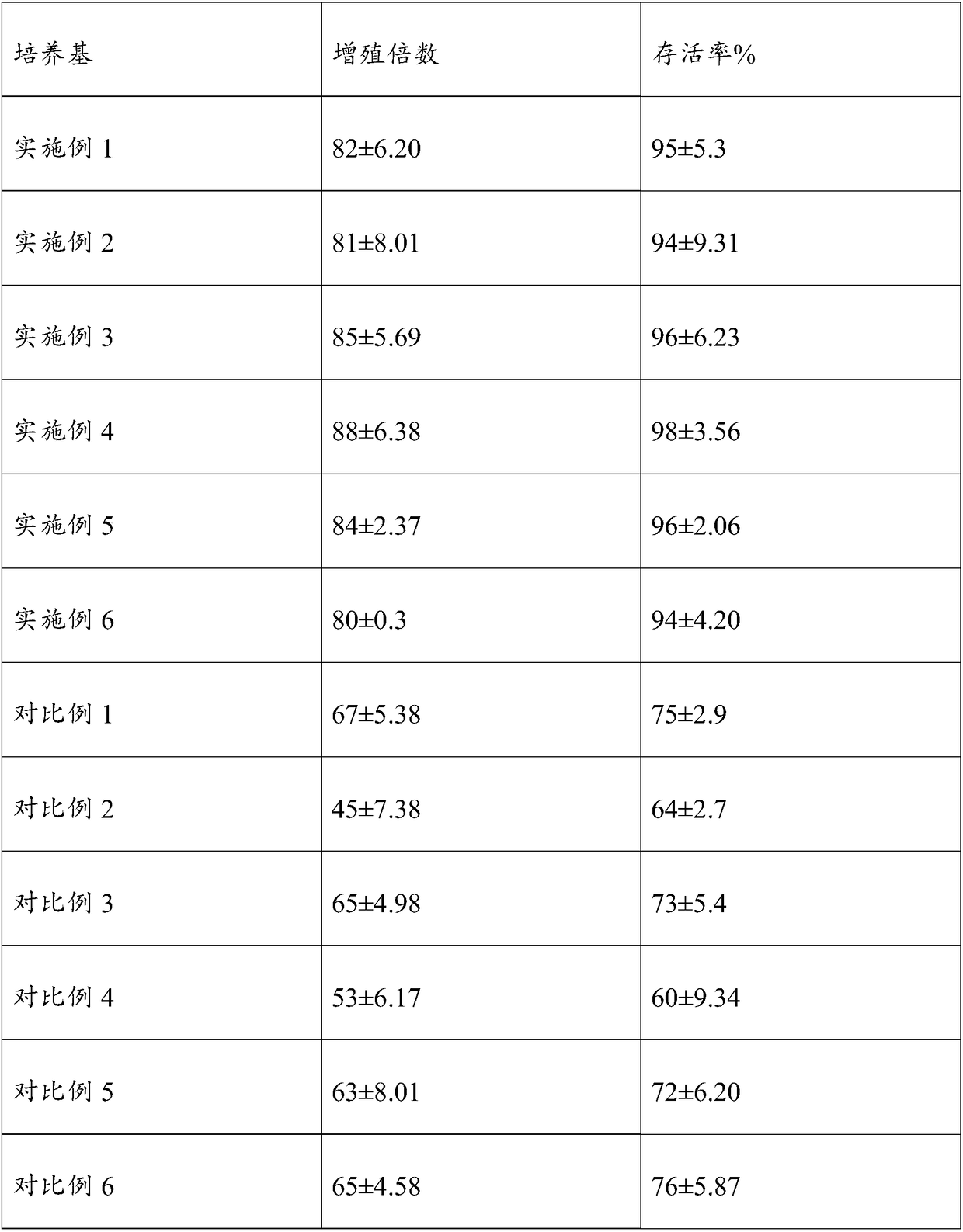
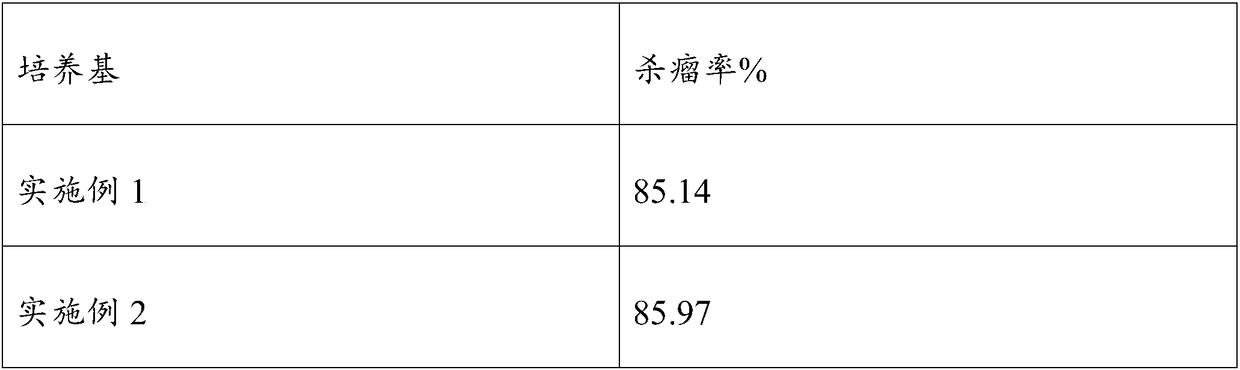
Embodiment 1
[0033] The serum-free cell culture medium of this embodiment mainly includes the following components, DMEM / F12 medium 80v / v%, sericin solution 50mg / ml, chitosan quaternary ammonium salt solution 2mg / ml, human blood white Protein 35mg / ml, phytohemagglutinin 30mg / ml, vitamin C 20ug / ml, vitamin E 40ug / ml, epidermal growth factor 1.0ng / ml, insulin-like growth factor 5ng / ml, L-glutamine 3mmol / ml.
[0034] The preparation method of the culture medium in this embodiment is as described above and will not be described here.
Embodiment 2
[0036] The serum-free cell culture medium of the present invention mainly includes the following components: DMEM / F12 medium 85v / v%, sericin solution 25mg / ml, chitosan quaternary ammonium salt solution 2.5mg / ml, human blood white Protein 50mg / ml, phytohemagglutinin 20mg / ml, vitamin C 50ug / ml, vitamin E 50ug / ml, epidermal growth factor 0.5ng / ml, insulin-like growth factor 10ng / ml, L-glutamine 1mmol / ml.
[0037] The preparation method of the culture medium in this example is the same as that in Example 1, and will not be described here.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com