Method for preparing ammonium hydrogen fluoride by circularly treating fluosilicic acid through sylvite
A technology for ammonium bifluoride and cyclic treatment, which is applied in the field of phosphorus chemical industry, can solve the problems of low one-time yield of ammonium bifluoride, high equipment requirements and investment, and restricted application, so as to reduce production costs, reduce production energy consumption, and operate with greater flexibility Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
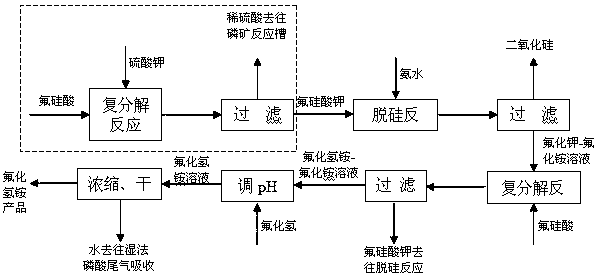
[0042] Add 220g of potassium fluorosilicate to ammonia water (347g) with a mass concentration of 20%, stir and react at 40°C for 40 minutes, let it stand for 10 minutes, and filter to obtain a silica filter cake and potassium fluoride-ammonium fluoride solution. The silicon filter cake is washed and dried to obtain a white carbon black product. The washing filtrate was combined with potassium fluoride-ammonium fluoride solution, and fluorosilicic acid (1200g) with a mass concentration of 12% was added, stirred and reacted at 40°C for 20 minutes, left to stand for 10 minutes, and filtered to obtain potassium fluorosilicate and ammonium bifluoride-fluoride ammonium chloride solution. Add hydrogen fluoride to the ammonium bifluoride-ammonium fluoride solution at 30°C to adjust the pH of the solution to 3 to obtain an ammonium bifluoride solution, which is concentrated and dried at an absolute pressure of 35kPa (drying temperature is 80°C) to obtain ammonium bifluoride product. ...
Embodiment 2
[0045] Add 220g of potassium fluorosilicate to ammonia water (463g) with a mass concentration of 15%, stir and react at 30°C for 30min, let it stand for 10min, and filter to obtain a silica filter cake and potassium fluoride-ammonium fluoride solution. The silicon filter cake is washed and dried to obtain a white carbon black product. The washing filtrate was combined with potassium fluoride-ammonium fluoride solution, and fluorosilicic acid (1030g) with a mass concentration of 14% was added, stirred and reacted at 40°C for 15 minutes, left to stand for 10 minutes, and then filtered to obtain potassium fluorosilicate and ammonium bifluoride-fluoride ammonium chloride solution. Add hydrogen fluoride to the ammonium bifluoride-ammonium fluoride solution at 50°C to adjust the pH of the solution to 3 to obtain an ammonium bifluoride solution, which is concentrated and dried at an absolute pressure of 80kPa (drying temperature is 100°C) to obtain ammonium bifluoride product.
[0...
Embodiment 3
[0048] Add 220g of potassium fluorosilicate to ammonia water (694g) with a mass concentration of 10%, stir and react at 70°C for 30min, let it stand for 10min, and filter to obtain a silica filter cake and potassium fluoride-ammonium fluoride solution. The silicon filter cake is washed and dried to obtain a white carbon black product. The washing filtrate was combined with the potassium fluoride-ammonium fluoride solution, and fluorosilicic acid (800g) with a mass concentration of 18% was added, stirred and reacted at 50°C for 30 minutes, left to stand for 10 minutes, and then filtered to obtain potassium fluorosilicate and ammonium bifluoride-fluorine ammonium chloride solution. Add hydrogen fluoride to the ammonium bifluoride-ammonium fluoride solution at 30°C to adjust the pH of the solution to 3 to obtain an ammonium bifluoride solution, which is concentrated and dried at an absolute pressure of 50kPa (the drying temperature is 90°C) to obtain ammonium bifluoride product....
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com