Method for preparing 3-chloropropane through catalytic oxidation of 1, 2-dichloropropane
A technology of dichloropropane and catalytic oxidation, which is applied in the preparation of dehydrohalogenation, chemical instruments and methods, preparation of halogenated hydrocarbons, etc., can solve problems such as certain side effects of biological growth, toxicity of dichloropropane, environmental pollution, etc., and achieve environmental protection. The effect of obvious benefits, solving processing problems and reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
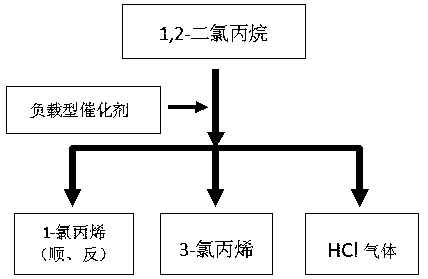
[0029] A method for preparing 3-chloropropene by catalytic oxidation of 1,2-dichloropropane, comprising the steps of:
[0030] (1) Put the metal carbonyl catalyst in a fixed-bed reactor, pass nitrogen gas, raise the temperature to 280°C, pass through 1,2-dichloropropane, carry out dehydrochlorination reaction at 300°C, and collect the reaction product to generate 3-chloro Propylene;
[0031] The gas released by the reaction is cooled to 50°C by the cooler, and then enters the fractionation tower. The temperature is controlled at 40°C, and the unreacted chlorinated hydrocarbons are cooled and separated. Materials for hydrochloric acid;
[0032] The metal carbonyl catalyst is a mixture of supported nickel tetracarbonyl and iron pentacarbonyl; during the dehydrochlorination reaction, the space velocity of nitrogen is 500h -1 ;
[0033] (2) The reaction product is washed with water, neutralized with alkali, and washed, then sent to a propylene compressor, cooled to become a liq...
Embodiment 2
[0036] A method for preparing 3-chloropropene by catalytic oxidation of 1,2-dichloropropane, comprising the steps of:
[0037] (1) Put the metal carbonyl catalyst in a fixed-bed reactor, pass nitrogen gas, raise the temperature to 320°C, pass through 1,2-dichloropropane, carry out dehydrochlorination reaction at 400°C, and collect the reaction product to generate 3-chloro Propylene;
[0038] The gas released by the reaction is cooled to 60°C by the cooler, and then enters the fractionation tower. The temperature is controlled at 50°C, and the unreacted chlorinated hydrocarbons are cooled and separated. The hydrogen chloride in the gas is washed and refined with water to obtain 35% hydrogen Materials for hydrochloric acid;
[0039] The metal carbonyl catalyst is a mixture of supported manganese decacarbonyl and cobalt octacarbonyl; during the dehydrochlorination reaction, the space velocity of nitrogen is 2000h -1 ;
[0040] (2) The reaction product is washed with water, neu...
Embodiment 3
[0043] A method for preparing 3-chloropropene by catalytic oxidation of 1,2-dichloropropane, comprising the steps of:
[0044](1) Put the metal carbonyl catalyst in a fixed-bed reactor, pass nitrogen gas, raise the temperature to 300°C, pass through 1,2-dichloropropane, carry out dehydrochlorination reaction at 350°C, collect the reaction product to generate 3-chloro Propylene;
[0045] The gas released by the reaction is cooled to 55°C by the cooler, and then enters the fractionation tower. The temperature is controlled at 45°C, and the unreacted chlorinated hydrocarbons are cooled and separated. Materials for hydrochloric acid;
[0046] The metal carbonyl catalyst is supported chromium hexacarbonyl; during the dehydrochlorination reaction, the space velocity of nitrogen is 1200h -1 ;
[0047] (2) The reaction product is washed with water, neutralized with alkali, and washed, then sent to a propylene compressor, cooled to become a liquid, and then cooled to below 10°C by a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com