Synthesis method of isoliquiritigenin
A technology of isoliquiritigenin and synthesis method, which is applied in the field of synthesis of isoliquiritigenin, can solve the problems of high cost, high requirements on reaction conditions, and low yield of isoliquiritigenin, and achieve low cost, simple and easy method, and reduced side effects. The effect of the risk of product formation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
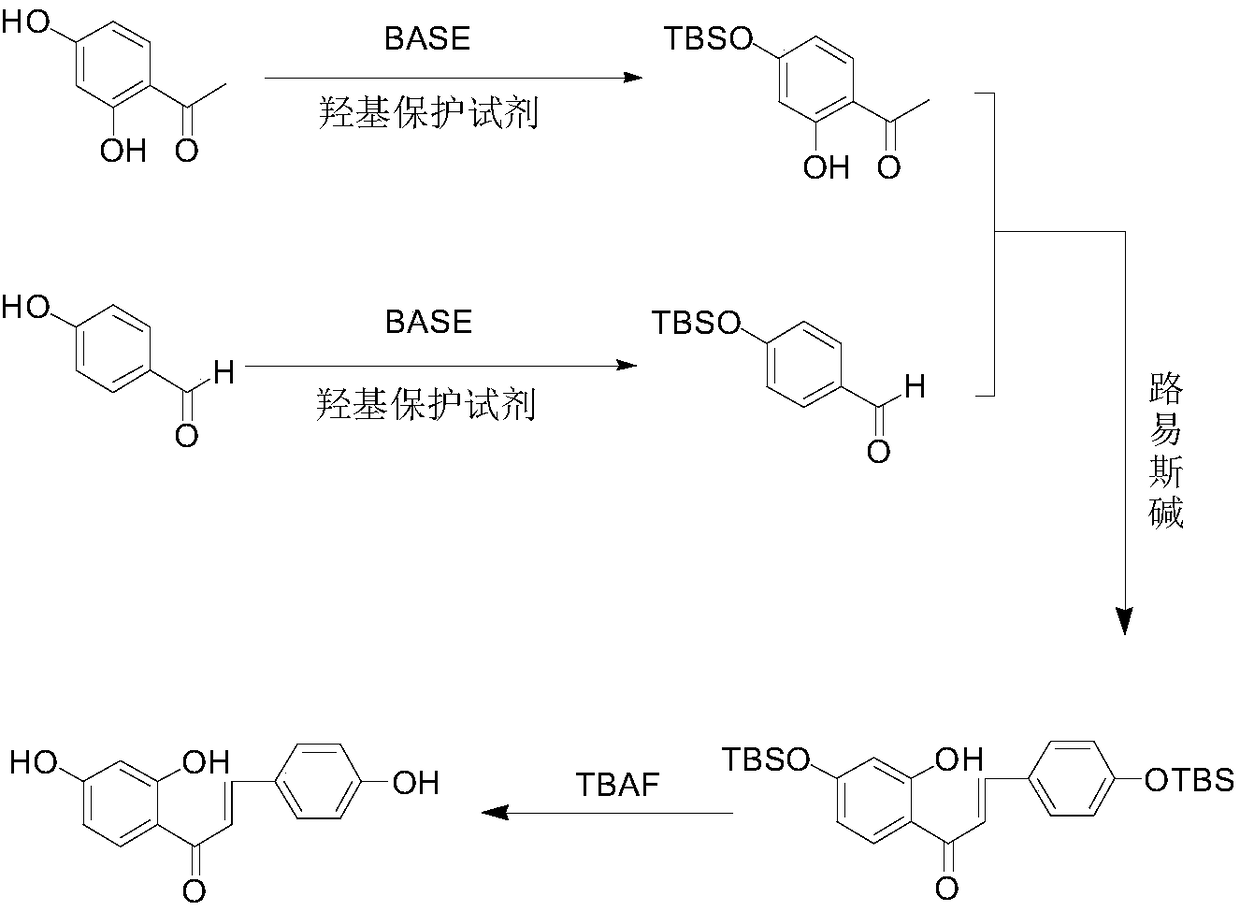
[0018] The embodiment of the present invention provides a synthesis method of isoliquiritigenin. The synthesis method of isoliquiritigenin uses 2,4-hydroxyacetophenone and 4-hydroxybenzaldehyde as raw materials, and undergoes hydroxyl protection, aldol condensation, and hydroxyl deprotection reactions to obtain the product isoliquiritigenin. The specific synthesis steps as follows:
[0019] (1) Provide 2,4-dihydroxyacetophenone, mix the 2,4-dihydroxyacetophenone with an alkaline reagent and an organic solvent, add a hydroxyl protection reagent, and stir the reaction at 0-100°C , protecting the 4-hydroxyl group of the 2,4-dihydroxyacetophenone;
[0020] (2) 4-hydroxybenzaldehyde is provided, after the 4-hydroxybenzaldehyde is mixed with an alkaline reagent and an organic solvent, a hydroxyl protection reagent is added, and a stirring reaction is performed at 0-100° C. Formaldehyde carries out hydroxyl protection;
[0021] (3) 2,4-dihydroxyacetophenone after step (1) hydroxyl...
Embodiment 1
[0043] A kind of synthetic method of isoliquiritigenin, concrete synthetic steps are as follows:
[0044] (1) Add 12.2g of 4-hydroxybenzaldehyde and 14.3g of imidazole into a round bottom flask, add dichloromethane and stir evenly, lower the system to 0-5°C, and slowly drop in 21.12g of dimethyl tert-butyl Chlorosilane, then stirred at 25-30°C, detected by TLC, after 6 hours the reaction was completed, water was added to the system, the organic phase was washed with 1N hydrochloric acid, and then washed with saturated brine, dried and distilled under reduced pressure to obtain 21.08 g of oily product, yield 89%.
[0045] (2) Add 13.71g of 2,4-dihydroxyacetophenone and 17.02g of imidazole into a round bottom flask, add dichloromethane and stir evenly, lower the system to 0-5°C, and slowly drop in 22.60g of dimethyl tert-butylchlorosilane, then stirred at 25-30°C, detected by TLC, after stirring for 5 hours, added water to the system, washed the organic phase with 1N hydrochlor...
Embodiment 2
[0049] A kind of synthetic method of isoliquiritigenin, concrete synthetic steps are as follows:
[0050] (1) Add 4.88g of 4-hydroxybenzaldehyde into a round bottom flask, add dichloromethane and stir to dissolve completely, then add 10.84g of diisopropylethylamine, cool the system to 0-5°C, slowly drop Add 10.75g of triisopropylchlorosilane, then stir at 25-30°C, detect by TLC, after 5h the reaction is complete, add water to the system, wash the organic phase with 1N hydrochloric acid, and then wash with saturated brine, the organic phase After drying, the solvent was distilled off under reduced pressure to obtain 9.68 g of oily product, with a yield of 87%;
[0051] (2) Add 4.56g of 2,4-dihydroxyacetophenone into a round bottom flask, add dichloromethane and stir to dissolve completely, then add 9.68g of diisopropylethylamine, and cool the system to 0-5 ℃, slowly drop 8.64g triisopropylchlorosilane, then stir at 25-30℃, TLC detection, after stirring for 4h, add water to the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com