NSAID sustained-release nanoparticles and preparation method thereof
A nanoparticle and active component technology, applied in the field of medicine, can solve the problems of slow drug penetration through the skin, side effects, etc., achieve good sustained release effect, good biocompatibility, and improve medication compliance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Example 1 Preparation of NSAID sustained-release nanoparticles (indomethacin)
[0055] 1 Materials and equipment
[0056] 1.1 Polymers, proteins and materials in general
[0057]
[0058]
[0059] 1.2 Solvent
[0060]
[0061] 1.3 Equipment
[0062]
[0063]
[0064] 2 specific preparation steps:
[0065] PLGA nanoparticles loaded with NSAID (indomethacin) were prepared by single emulsion technique. 40 mg NSAID was dissolved in 2 ml PLGA (RG 504) in ethyl acetate (100 mg / ml). Then 4 ml of aqueous PVA (1% w / v) was added and the resulting o / w emulsion was sonicated on ice. The emulsion was diluted with 90 mL of PVA solution (0.3% w / v), and the solvent was quickly removed by rotary evaporation. Finally, the particles were isolated by centrifugation at 10,000 g (Beckman, Model J2-21 centrifuge) for half an hour and washed three times with water at the same centrifugation speed and processing time. Dried particles were collected by freeze drying.
[00...
Embodiment 2
[0071] Embodiment 2 prepares NSAID sustained-release nanoparticles (ketoprofen)
[0072] The specific preparation method is the same as in Example 1, except that indomethacin is replaced by ketoprofen.
Embodiment 3
[0073] Example 3 Characterization of General Evaluation Methods
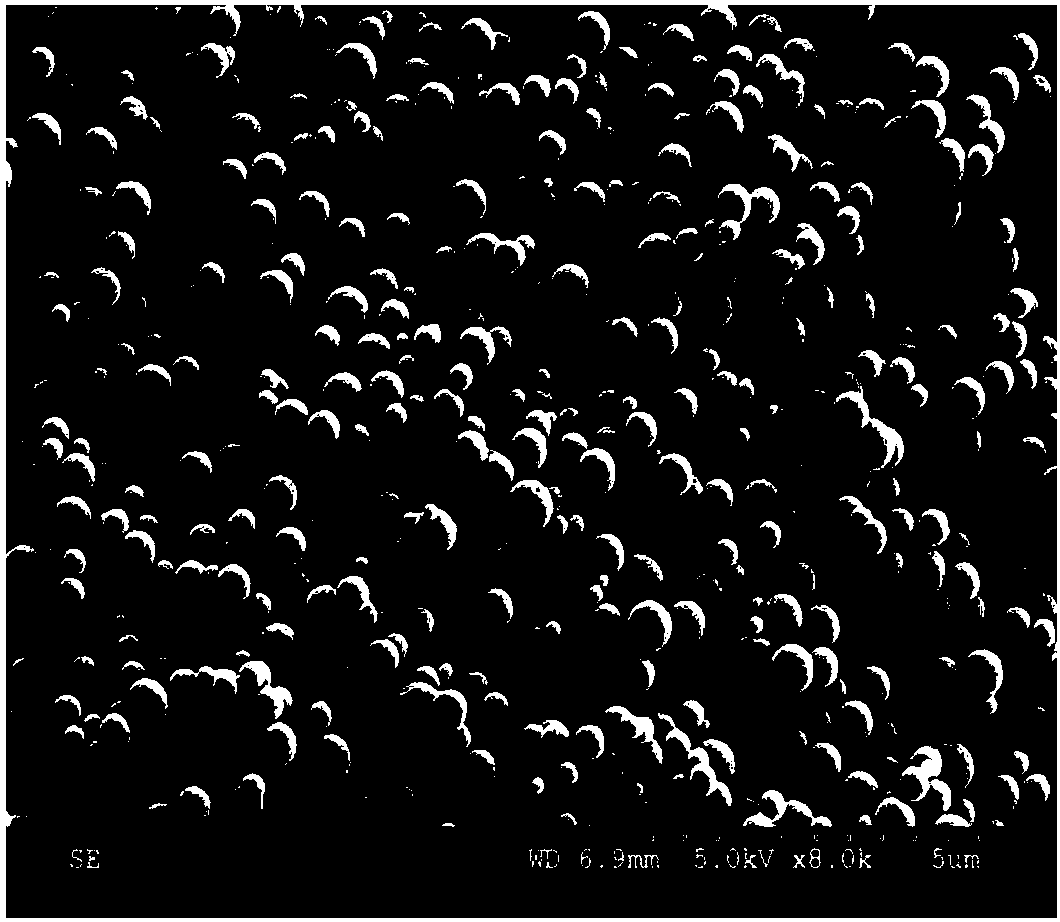
[0074] 1. Characterization of nanoparticles
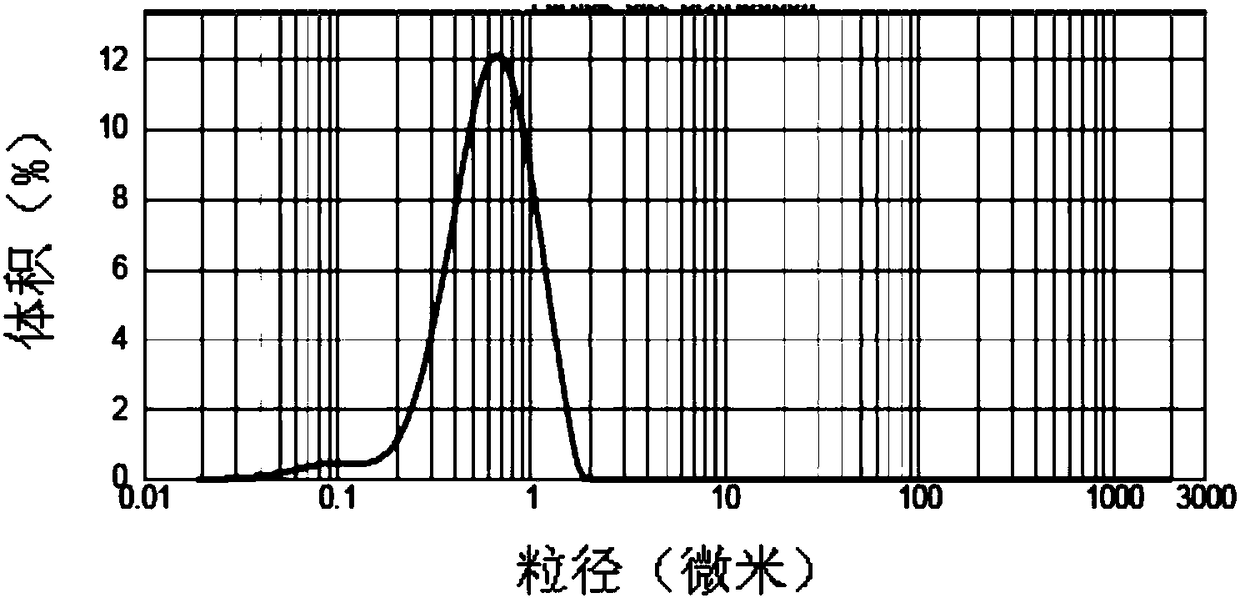
[0075] Laser diffraction analysis: Malvern Mastersizer 2000 was used to measure the particle size, and the Hydroμp sample dispersion unit was connected. 5-10 mg microparticles were dispersed in 1 ml deionized water and sonicated for 5 minutes before adding to the sample. The sample was added to the Mastersizer measuring cell, and the measurement process was controlled by Malvern Mastersizer 2000 software. Particle sizes were plotted as particle size distribution (by volume) and span values were calculated. Span is a measure of the width of the volume distribution relative to the median diameter and is calculated as (D90%-D10%) / D50%. D90%, D50% and D10% are particle diameters measured at 90%, 50% and 10% volume distribution of cumulative particles, respectively. Samples were measured in triplicate.
[0076] Light Scattering Analysis
[0077] Particle size was ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com