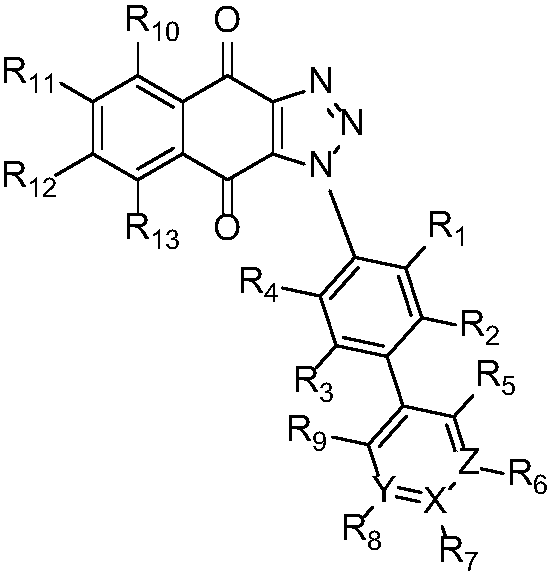
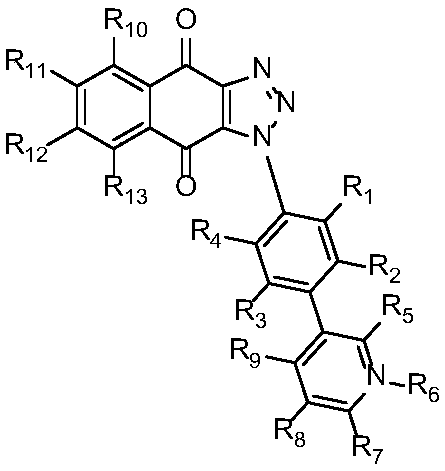
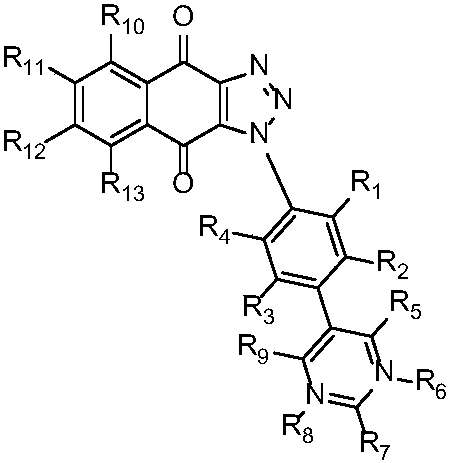
Triazole naphthoquinone connected aromatic (heterocyclic) ring derivative
A technology of triazole naphthalene and its derivatives, which is applied in the field of chemical medicine and can solve the problems of limited anti-tumor activity and large side effects of bone marrow suppression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1, Preparation of 3-methoxy-[1,1'-biphenyl]-4-amino (intermediate 1a)
[0030]
[0031] 4-bromoaniline 1g (5.81mmol) and m-methoxyphenylboronic acid 883mg (5.81mmol.1eq) potassium carbonate (4.82g 34.88mmol 6eq) [1,1'-bis (diphenylphosphino) ferrocene ] Palladium dichloride (425.35mg 581mmol6eq) was placed in a 100ml two-neck bottle, injected with 30ml (dioxane: water = 3:1) under nitrogen protection, and reacted at 80 for 3.5h. After the reaction is monitored by TLC, post-processing vacuum distillation to the remaining about 10ml, the solution is poured into a separatory funnel, extracted 2-3 times with dichloromethane, dried with anhydrous Na2SO4, filtered, vacuum distilled and spin-dried to obtain black oily droplets , separated and purified by silica gel column chromatography with petroleum ether:ethyl acetate=6:1 to obtain about 720 mg of light yellow oily liquid with a yield of 62%.
[0032] 1 H-NMR (400MHz, CDCl 3 ):8.02–7.94(m,4H),7.46(t,J=7.9Hz,1H...
Embodiment 2
[0033] Example 2, Preparation of 3-trifluoromethoxy-[1,1'-biphenyl]-4-amino (intermediate 1b)
[0034]
[0035] 4-Bromoaniline 1g (5.81mmol) and m-trifluoromethoxyphenylboronic acid 1200mg (5.81mmol.1eq) potassium carbonate (4.82g 34.88mmol 6eq) [1,1'-bis (diphenylphosphino) di Ferrocene]palladium dichloride (425.35mg 581mmol6eq) was placed in a 100ml two-neck flask, injected with 30ml (dioxane:water=3:1) under nitrogen protection, and reacted at 80°C for 3.5h. After the reaction is monitored by TLC, post-processing vacuum distillation to the remaining about 10ml, the solution is poured into a separatory funnel, extracted 2-3 times with dichloromethane, dried with anhydrous Na2SO4, filtered, vacuum distilled and spin-dried to obtain black oily droplets , separated and purified by silica gel column chromatography with petroleum ether:ethyl acetate=6:1 to obtain about 780 mg of light yellow oily liquid with a yield of 53%.
[0036] 1 H-NMR (400MHz, CDCl 3 ):8.02–7.94(m,4H)...
Embodiment 3、3
[0037] Example 3, Preparation of 3.5-difluoro-(3-trifluoromethoxy-[1,1'-biphenyl])-4-amino (intermediate 1c)
[0038]
[0039]4-Bromo-3.5-difluoro-aniline 1g (4.81mmol) and m-trifluoromethoxyphenylboronic acid 990mg (4.81mmol.1eq) potassium carbonate (4.82g 24.88mmol 6eq) [1,1'-bis(di Phenylphosphino)ferrocene]palladium dichloride (425.35mg 481mmol 6eq) was placed in a 100ml two-necked flask, injected with 30ml (dioxane: water = 3:1) under nitrogen protection, and reacted at 80°C for 3.5h. After the completion of the reaction monitored by TLC, post-processing vacuum distillation to the remaining about 10ml, the solution was poured into a separatory funnel, extracted 2-3 times with dichloromethane, dried with anhydrous Na2SO4, filtered, vacuum distilled and spin-dried to obtain black oily droplets , separated and purified by silica gel column chromatography with petroleum ether:ethyl acetate=6:1 to obtain about 680 mg of light yellow oily liquid with a yield of 49%.
[0040...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com